A recent study by Prof. QIAN Youcun’s group from Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS) found that food-derived nucleic acids could maintain the homeostasis of small intestinal natural intraepithelial lymphocytes (IELs), thereby promoting protein-mediated oral tolerance establishment. Entitled “Dietary nucleic acids promote oral tolerance through innate sensing pathways in mice”, this research was published online in Nature communications on Nov. 1, 2024.
The intestine is not only essential for digestion and nutrient absorption but also serves as the body’s largest immune organ. Intraepithelial lymphocytes (IELs), a unique type of immune cell within the intestinal mucosal epithelium, express distinct TCR receptors and various combinations of molecular markers such as CD4, CD8α, and CD8β. IELs play crucial roles in maintaining immune balance within the intestine, safeguarding epithelial barriers, and participating in immune responses. Unlike traditional T cells, IELs rely heavily on the intestinal mucosal microenvironment for their development. While the development of induced IELs is primarily influenced by the intestinal flora, the regulatory factors governing the more abundant natural IELs remain largely unknown. Additionally, oral tolerance is an immune process in which the immune system becomes unresponsive, or "tolerant", to antigens encountered in the digestive tract. Previous research has shown that multiple types of immune cells are involved in establishing oral tolerance. However, it remains unclear whether natural IELs also play a role in this process.
In this study, researchers systematically analyzed IEL development in mice under steady-state conditions with deficiencies in nucleic acid receptors and key downstream adaptor proteins. They found that in mice with double deletion of MAVS and STING (MS DKO), the proportion and number of two groups of natural IELs (TCRγδ+ and TCRαβ+CD8αα+) in the small intestine were significantly reduced. Further analysis in germ-free mice revealed that the development of these cells was not dependent on the intestinal flora. Using a synthetic diet, researchers discovered that the development of natural IELs relied on dietary nucleic acids. Mechanistically, dietary nucleic acids influenced the development of natural IELs by modulating IL-15 expression in intestinal epithelial cells.
Subsequently, researchers further found that OVA-mediated oral tolerance could not be established in MS DKO mice or in mice fed a synthetic diet. However, restoring natural IELs facilitated tolerance. Further investigation showed that natural IELs could secrete Tgfb1 to promote CD103+ DCs to express integrin β8, which is essential for CD103+ DCs to drive antigen-specific Treg differentiation. Finally, by reconstituting Tgfb1-deficient IELs, they confirmed the indirect regulatory role of IELs on OVA-specific Tregs.
In summary, this study discovered that dietary nucleic acids play a critical role in promoting the development and maintenance of natural IELs in the small intestine of mice under steady-state conditions. By identifying dietary nucleic acids as a necessary factor for natural IEL survival, this research highlights the unique role of diet beyond nutrition, showing its involvement in immune cell homeostasis. Additionally, the study revealed that these natural IELs contribute significantly to protein antigen-mediated oral tolerance, which deepens the understanding of the broader mechanisms underlying intestinal immune tolerance.
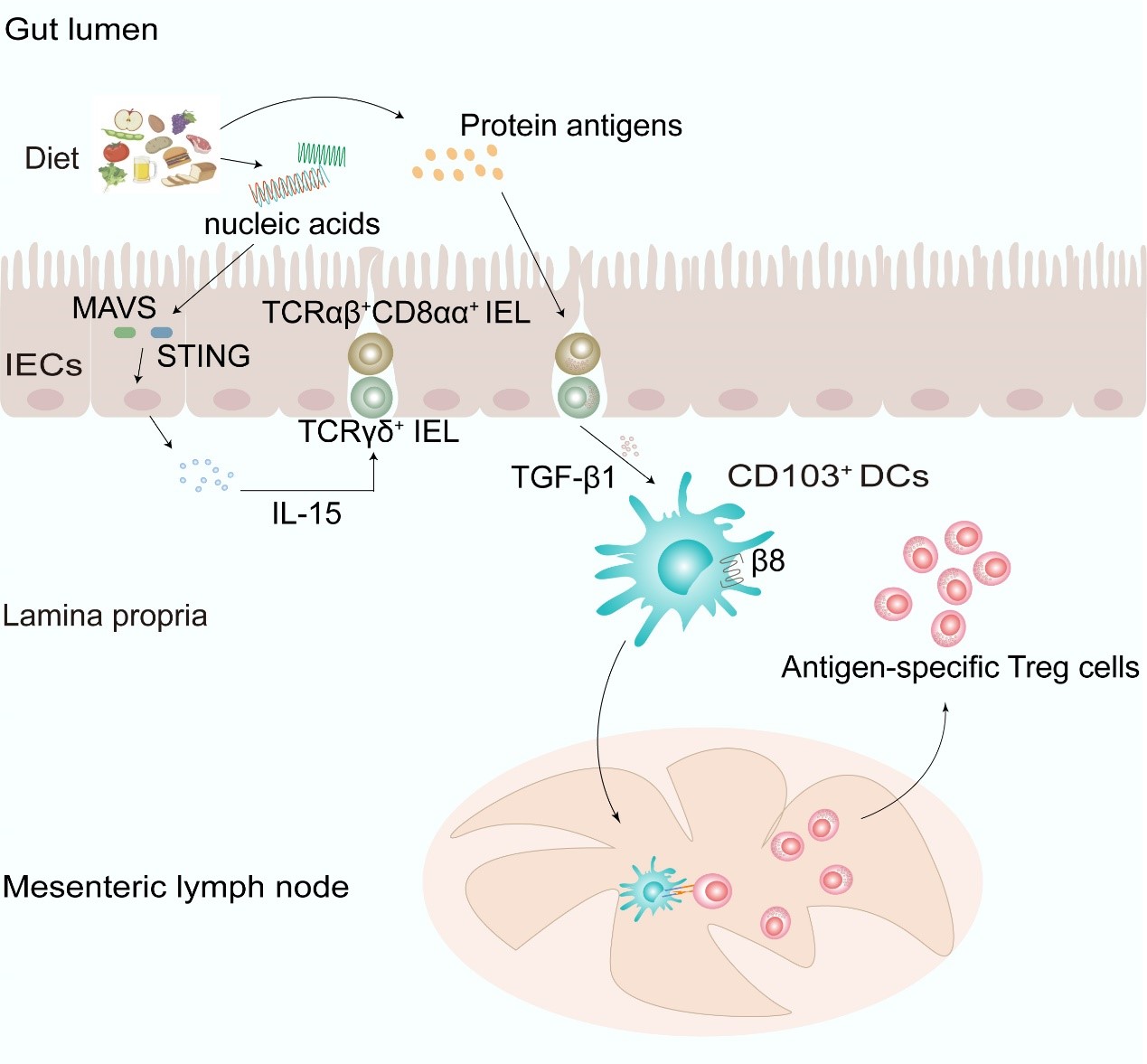
Dietary nucleic acids promote the establishment of oral tolerance by regulating the homeostasis of innate intraepithelial lymphocytes in the small intestine. (Image provided by Prof. QIAN Youcun’s team)
This project was financially supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China. This work was also supported and assisted by Prof. QIU Ju and Prof. HU Guohong from SINH, Prof. CHEN Jianfeng and Prof. SONG Xinyang from the Center for Excellence in Molecular Cell Biology, CAS, and Prof. LENG Qibin from Guangzhou Medical University.
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

