A research article describing the genomic and transcriptomic landscape of gastrointestinal stromal tumor, entitled “Genomic and Transcriptomic Landscape of Human Gastrointestinal Stromal Tumors” was published online in the scientific journal Nature Communications on Nov. 3, 2024.
Although gastrointestinal stromal tumor (GIST) is the most common type of human sarcoma, it was not included in The Cancer Genome Atlas (TCGA) sarcoma project. GISTs span a biological behavior spectrum that range from benign to malignant/advanced state, with some small GIST lesions remaining stable for years whereas others progress rapidly to widespread metastatic disease. Therefore, GISTs provide an ideal model by which to study constraints to tumorigenic progression. The opportunity to study benign lesions such as early-stage GIST enables evaluations of the sequence of mutations accounting for oncogenic progression. Such studies are more challenging in sarcomas and other cancers where a benign state cannot be identified. Due to the limited numbers of GISTs that have been profiled and analyzed, molecular events driving progression and subtypes are not firmly established.
A research team jointly led by Prof. WANG Yuexiang from Shanghai Institute of Nutrition and Health of Chinese Academy of Sciences, Prof. CAO Hui from Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, and Prof. WU Kui from Institute of Intelligent Medical Research, BGI Genomics comprehensively described the genomic and transcriptomic landscape of a cohort of 117 GISTs including early-stage, curable tumors and advanced, lethal cancers from 105 patients, comprising the largest-to-date cohort of GISTs sequenced.
This study provides a blueprint of the genetic alterations responsible for clinical progression from early-stage to advanced GISTs. The findings also advance the understanding of molecular subtypes, subdivide 4 subtypes with different genomic, immune and clinical characteristics, and provide potential insights to guide subtype-specific treatment strategies. For example, GIST patients with the C2 subtype (CD8+ inflamed subtype, intestinal GISTs) might ultimately have a potential response to immunotherapy while the patients with the C3 (immune desert subtype, gastric GISTs) may not benefit from immunotherapy, but are potential candidates for treatment with CDK4/6 inhibitors. This large collection of comprehensively profiled GISTs with clinical information enables a deeper understanding of the molecular landscape of GISTs and offers an opportunity for more precise treatment.
This project was financially supported by the National Natural Science Foundation of China, the National Key Research and Development Program of China, the Innovation Program of Shanghai Science and technology committee, and the Postdoctoral Fellowship Program of CPSF.
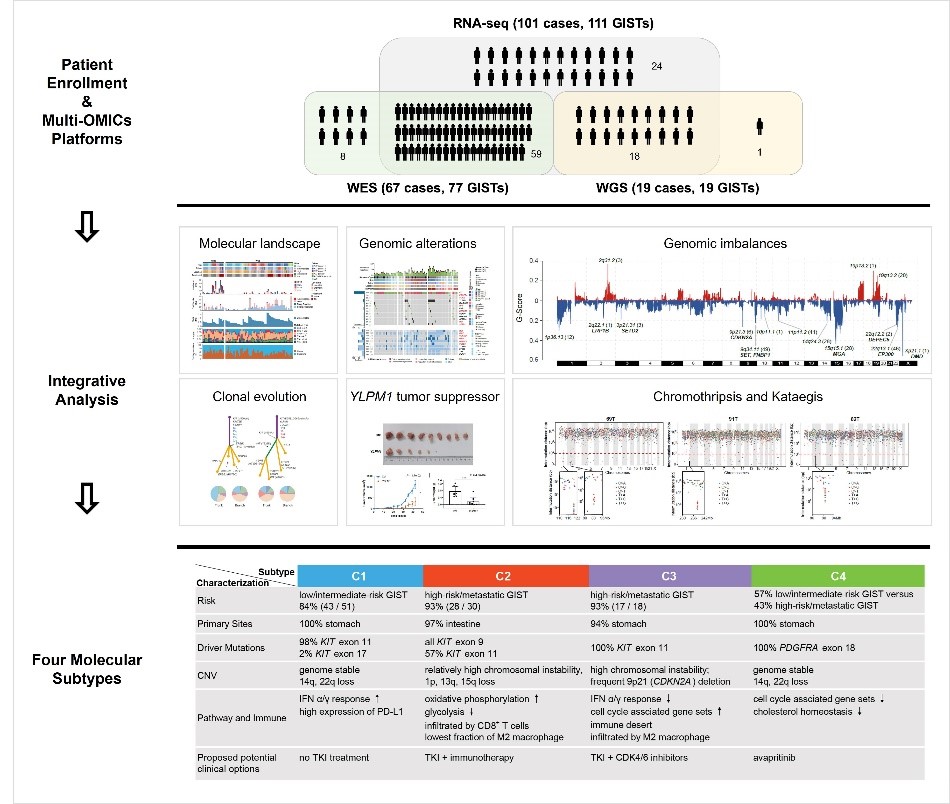
Genomic and transcriptomic landscape of GIST, four molecular subtypes and potential subtype-specific treatment strategies. (Image provided by Prof. WANG Yuexiang’s group)
Media Contact:
WANG Jin (Ms.)
Shanghai Institute of Nutrition and Health
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

