On March 13, 2025 (Beijing Time), the research team led by Minrui Fan from the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, in collaboration with Xudong Wu’s team from Westlake University, Jinru Zhang’s team from Fudan University, and Nannan Su’s team from Zhejiang University, published a research paper titled "Structure and mechanism of the plastid/parasite ATP/ADP translocator" in the internationally renowned academic journal Nature. In the study, the researchers determined high-resolution cryo-electron microscopy structures of ATP/ADP translocators in chloroplasts and Chlamydia, deepening our understanding of the molecular mechanisms behind transmembrane energy transfer during chloroplast endosymbiosis.
As we all know, mitochondria are the powerhouses of eukaryotic cells, producing the energy (ATP) required for life activities. Within the inner membrane of mitochondria, there is a type of ATP/ADP translocator known as AAC, which transfers ATP from the mitochondria to the cytoplasm to provide energy for metabolic activities while transporting ADP from the cytoplasm into the mitochondria for ATP synthesis. Research has found that the inner membranes of plant cell chloroplasts (and other plastids, such as amyloplasts for starch synthesis) also contain an ATP/ADP translocator called NTT. Interestingly, NTT functions in the opposite manner to mitochondrial AAC proteins. NTT transports ATP from the cytoplasm into the chloroplast to supply energy for metabolic activities while transferring ADP and phosphate (Pi) from the chloroplast back into the cytoplasm. Compared to mitochondrial AAC proteins, chloroplast NTT is the most substrate-specific ATP/ADP translocator known. It can only transport ATP/ADP and cannot transport other nucleotide types, such as GTP/GDP, CTP/CDP, or deoxynucleotides like dATP/dADP. Studies have shown that plastid NTT plays a crucial role in substance and energy metabolism processes, including plant photosynthesis, starch synthesis, and fatty acid synthesis. Notably, homologous proteins of NTT in diatoms exhibit substrate diversity. They can transport various intracellular nucleotides as well as artificially designed non-natural nucleotides, making them highly valuable for applications in synthetic biology.
Interestingly, similar ATP/ADP translocators to NTT are also present on the cell membranes of obligate intracellular pathogens such as Chlamydia. These proteins are responsible for transferring ATP from the host cell into the pathogen’s cell to provide energy for its metabolism, a phenomenon known as energy parasitism. In contrast, free-living bacteria, such as Escherichia coli and cyanobacteria, do not possess NTT proteins. If cyanobacteria lack NTT proteins, yet chloroplasts—believed to have evolved from cyanobacteria according to the endosymbiotic theory—contain them, then where did chloroplast NTT proteins originate? One hypothesis suggests that during the endosymbiotic process, an ancestral eukaryotic cell engulfed both a nitrogen-fixing cyanobacterium and a Chlamydia-like bacterium simultaneously. Since nitrogen fixation requires a significant amount of energy, the cyanobacterium may have acquired the ATP-transporting NTT protein from Chlamydia through horizontal gene transfer. This protein was subsequently retained throughout evolution and eventually became an essential component of chloroplasts and other plastids. Notably, recent research has introduced NTT proteins into cyanobacteria, enabling them to establish a symbiotic relationship with energy metabolism-deficient yeast cells in vitro. This engineered endosymbiosis allowed the cyanobacteria to co-survive with the yeast cells for up to 24 generations.
To verify the origin of the chloroplast NTT protein and elucidate the molecular mechanism of its recognition and transport of ATP/ADP, the research group led by Minrui Fan and collaborators employed cryo-electron microscopy combined with nanobody assistance to determine high-resolution three-dimensional structures (2.72 Å-2.90 Å) of NTT proteins from Arabidopsis chloroplasts and Chlamydia pneumoniae. The three structures of the Arabidopsis chloroplast NTT protein were found in three conformations: an outward-open conformation and two substrate-bound inward partially open conformations. The two structures of the Chlamydia pneumoniae NTT protein were found in an outward-open conformation and an ATP-bound inward-open conformation. The structure revealed that the NTT protein consists of 12 transmembrane helices, adopting a fold typical of the MFS (major facilitator superfamily) superfamily transporters. The first six transmembrane helices form the N-terminal domain, while the last six transmembrane helices form the C-terminal domain. Structural superposition of the NTT proteins from Arabidopsis chloroplasts and Chlamydia pneumoniae showed that despite significant species-specific differences, the two proteins have highly similar three-dimensional structures. This finding supports the hypothesis that the chloroplast NTT protein in plants is derived from the corresponding protein in Chlamydia species.
The study found that the binding site for ATP (or ADP and Pi) is located in the central region of the NTT protein, between the N-terminal and C-terminal domains. The binding of ATP involves extensive interactions between its three moieties (adenine, ribose, and phosphate) and the NTT protein. The adenine portion of ATP is sandwiched between aromatic and hydrophobic amino acid residues, while its negatively charged phosphate groups interact with surrounding positively charged amino acid residues. The binding site for ADP is similar to that of ATP, but there are some conformational differences: the phosphate groups of ATP adopt an extended conformation, while the phosphate groups of ADP are folded, leading to slightly different surrounding interacting residues. Interestingly, the binding position for Pi corresponds exactly to the position of ATP’s γ-phosphate group. To validate these structural findings, the researchers performed ATP/ADP exchange experiments based on chemiluminescence and ATP-32P uptake experiments using radioactive isotopes. A series of mutants at the ATP/ADP binding site were designed, and the transport activity measurements confirmed the structural findings. Moreover, the researchers also analyzed the thermal stability of the NTT protein and found that Pi significantly enhanced ADP binding, suggesting a cooperative effect between the two. This finding aligns with the co-transport properties of ADP and Pi in NTT. Additionally, the study revealed that when an evolutionarily conserved asparagine residue (N282 in Arabidopsis AtNTT1) in NTT is mutated to alanine, the transport activity towards other nucleotides (such as GTP, CTP, and UTP) significantly increased. This suggests that this residue may play a crucial role in the specific recognition of ATP by NTT.
On the other hand, by comparing the structures of chloroplast and Chlamydia NTT proteins in different conformations, along with mutational and functional experiments, the study reveals that the N-terminal and C-terminal domains of NTT are two relatively rigid parts, and these domains exhibit relative movement between them, and by altering the interactions between the two domains, the protein facilitates the binding, transmembrane transport, and release of ATP or ADP plus Pi. This transport mechanism aligns with the "rocker-switch" alternating pathway model of the MFS superfamily. In this model, the conformational changes between the N-terminal and C-terminal domains allow the protein to alternately open and close its transport pathway, ensuring the efficient binding and release of the substrate and facilitating its translocation across the membrane. This mechanism is crucial for NTT's ability to carry out its role in ATP/ADP exchange and the co-transport of Pi.
This study not only unveils the molecular mechanism of substrate recognition and transmembrane transport by the chloroplast and Chlamydia ATP/ADP translocator NTT, but also deepens our understanding of the transmembrane energy transfer mechanism during the endosymbiotic process of chloroplasts. Additionally, it provides valuable insights for engineering NTT to improve crop yield and for designing NTT inhibitors as potential drugs to treat diseases caused by obligate intracellular pathogens.
The study was co-authored by Huajian Lin, research associate at the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Jian Huang, Ph.D. student at Westlake University, Tianming Li, research assistant at the Center for Excellence in Molecular Plant Sciences, and Wenjuan Li, postdoctoral researcher, as co-first authors. The corresponding authors are Minrui Fan, Principal Investigator at the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Xudong Wu, Principal Investigator at Westlake University, Jinru Zhang, Principal Investigator at Fudan University, and Nannan Su, Principal Investigator at Zhejiang University. The study also involved contributions from Yutong Wu and Tianjiao Yang, Ph.D. students at the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, Yuwei Nian, postdoctoral researcher, Ruiying Wang, and Xiaohui Zhao, research assistants, Xiang Lin, Master’s student at Fudan University, and Jiangqin Wang, postdoctoral researcher at Zhejiang University. The researchers thank the cryo-electron microscopy facilities of Zhejiang University, Westlake University, and Fudan University, as well as the public technical service center of the Center for Excellence in Molecular Plant Sciences for support and assistance. Special thanks are given to Naixu Xu and Genyun Chen from the isotopes laboratory of the Center for Excellence in Molecular Plant Sciences for their help. This study was supported and funded by the Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences, the Strategic Priority Research Program of the Chinese Academy of Sciences, the Shanghai Branch of the Chinese Academy of Sciences, and the "Science and Technology Innovation Action Plan" of Shanghai.
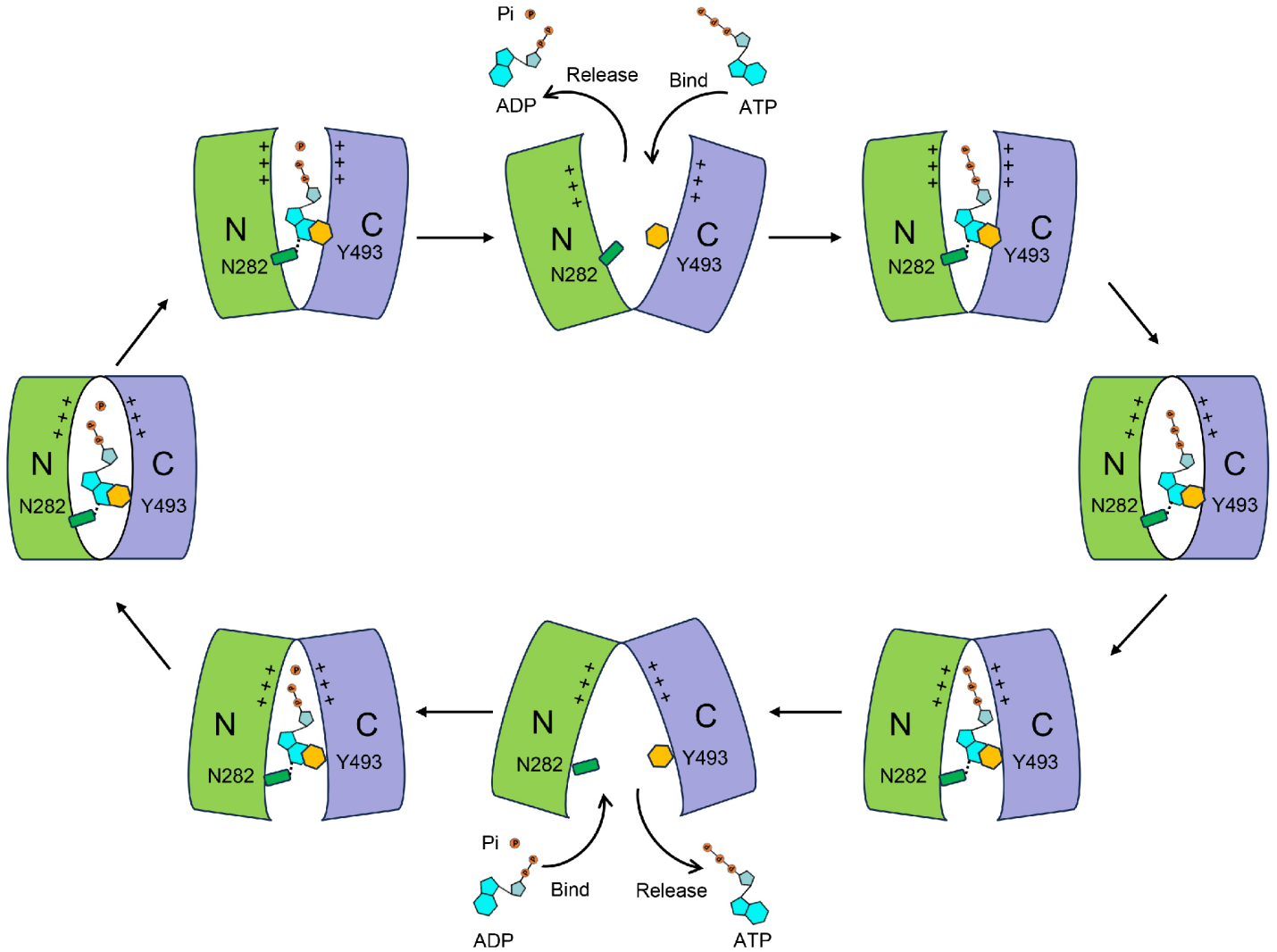
Fig1. NTT-mediated ATP/ADP translocation across membrane
Link to paper: https://www.nature.com/articles/s41586-025-08743-3
Contact:
Dr. Minrui Fan, Professor
National Key Laboratory of Plant Molecular Genetics, CAS Center for Excellence in Molecular Plant Sciences
Email: mrfan@cemps.ac.cn

