A research team led by Prof. LI Yu at the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences, identified a novel mechanism in understanding the therapeutic mechanism of Fibroblast Growth Factor 21 (FGF21) in treating liver fibrosis and metabolic dysfunction-associated steatohepatitis (MASH) via the protein phosphatase PPP6C. Entitled “Protein phosphatase 6 regulates metabolic dysfunction-associated steatohepatitis via the mTORC1 pathway”, this study was published online in Journal of Hepatology on Feb 10, 2025.
MASH, a global health concern, significantly increases the risk of liver cirrhosis and hepatocellular carcinoma. While FGF21 analogs have shown promise in clinical trials, their underlying molecular mechanisms remained unclear until now.
By establishing a diet-induced MASH model, the researchers found that hepatocyte-specific knockout of βKlotho significantly blocked the improvement of MASH by FGF21, confirming that FGF21 ameliorates MASH progression through the autocrine signaling pathway mediated by the FGFR/βKlotho receptor complex in hepatocytes.
Furthermore, the researchers employed protein interaction mass spectrometry screening and identified that the protein phosphatase PPP6C can directly bind to βKlotho. The study found that the absence of PPP6C blocked the therapeutic effects of FGF21 on MASH.
Mechanistically, the FGF21/βKlotho signaling pathway activates PPP6C phosphatase activity, which recruits and mediates the dephosphorylation of TSC2 at Ser939 and Thr1462, thereby inhibiting mTORC1 activity and promoting the nuclear translocation of transcription factors TFE3 and Lipin1.
Importantly, PPP6C expression levels were significantly reduced in liver tissues from both clinical patients and mouse models of MASH, indicating that PPP6C may act as a negative regulator of MASH progression in mice and humans.
The study demonstrated that pharmacological administration of FGF21 protects against MASH pathology at least in large through the interaction between βKlotho and PPP6C and PPP6C-mediated dephosphorylation of TSC2 in hepatocytes.
These findings advance the understanding of FGF21 signals in hepatocytes and demonstrate that targeting PPP6C may have therapeutic potential for treating liver fibrosis and MASH.
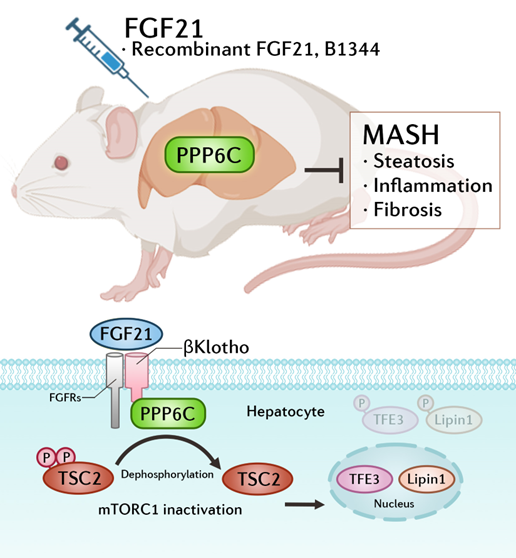
The proposed model for FGF21-activated PPP6C in treating liver Fibrosis and metabolic dysfunction-associated steatohepatitis. Pharmacological administration of FGF21 ameliorates MASH through an autocrine mechanism mediated by βKlotho-PPP6C interaction, and PPP6C-mediated dephosphorylation of TSC2 in hepatocytes. (Image by Prof. LI Yu's group)
Scientific Contact:
LI Yu, Ph.D.,
Professor
CAS Key Laboratory of Nutrition, Metabolism and Food Safety
Shanghai Institute of Nutrition and Health
Chinese Academy of Sciences
Email: liyu@sinh.ac.cn
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

