The glaucogenin family, which is a collection of unique 13,14:14,15-disecopregnane steroids, exists mainly in some Chinese herbal medicines, such as “Bai-Qian”, "Bai-Wei”, and “Xu-Chang-Qing” , which all belong to the Asclepiadaceae plant family. Recently, HAO Xiaojiang et al. of Kunming Institute of Botany, CAS, found that glaucogenin C and its glycosides were effective and selective inhibitors of alpha-virus-like positive-strand RNA viruses including plant-infecting tobacco mosaic virus (TMV) but not of other RNA or DNA viruses, yet they showed no toxicity to host cells (IC50 = 17 nM) (Proc. Natl. Acad. Sci. 2007, 104, 8083). Thus, they have the potential as novel antiviral agents. Despite the unique chemical structure and wonderful medicinal prospect of the glaucogenin family, no chemical synthesis of these steroids has been reported to date.
On the basis of the possible biosynthetic mechanism of the nine-membered lactone ring construction, the research group of Prof. TIAN Weisheng of Key Laboratory of Synthetic Chemistry of Natural Substances at Shanghai Institute of Organic Chemistry (SIOC), CAS, developed an efficient synthetic strategy to construct the key nine-membered lactone ring via Schenck ene reaction and subsequent iron(II)-promoted regioselective fragmentation reaction of a-alkoxy hydroperoxide. In this way, they finished the preparation of the common synthetic intermediate of glaucogenin family and the first synthesis of 5,6-dihydro-glaucogenin C (Angew. Chem. Int. Ed. 2011, 50, 7093).
This research work was listed as "Research Highlights" in Nature Chemical Biology (Nat. Chem. Biol. 2011, 7, 496) shortly after its publication, which stated that these results not only provided support for the biosynthetic proposal of glaucogenin family, but also offered a highly efficient route to the key synthetic intermediate. The Schenck ene reaction in tandem with the radical trapping protocol in macrolide synthesis was regarded as the highlight of this work.
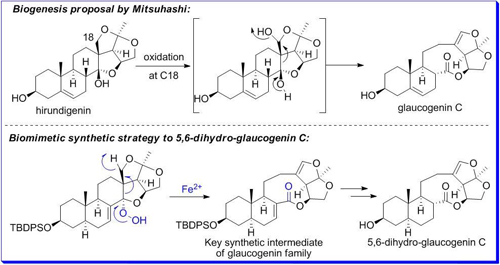
The efficient synthetic strategy to construct the key nine-membered lactone ring of 5,6-dihydro-glaucogenin C.
(Image by Prof. TIAN’s group)

