Clp protease (ClpP) plays a critical role in various processes that regulate cellular functions via proteolysis in both prokaryotes and eukaryotes. Dr. JIANG Hualiang's group and Dr. YANG Caiguang's group in Shanghai Institute of Materia Medica (SIMM) have collaborately made a great breakthrough in understanding the regulation mechanisms of ClpP from Staphylococcus aureus.
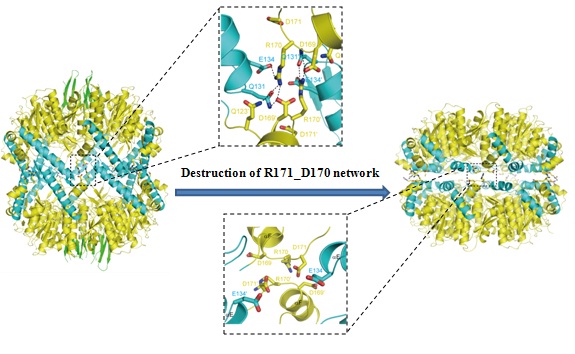 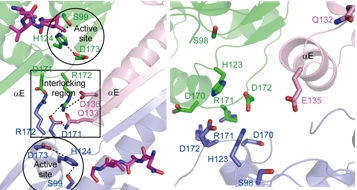 |
| Figure. The molecular basis of the regulation mechanism of ClpP (Image by SIMM) |
Two Ph.D. candidates ZHANG Jie and YE Fei, supervised by Dr. YANG Caiguang and Dr. LUO Cheng, demonstrated the enzymatic dynamics and acting mechanisms of ClpP based on theoretical and experimental approaches. ClpP, which consists of two heptameric rings that enclose a large chamber, plays an essential role in proteolysis bacterial. It has been reported that a S. aureus ClpP knockout strain displayed significantly reduced pathogenesis by 1000 times. In this study, they first solved the crystal structures of ClpP from Staphylococcus aureus in two distinct states: the extended state and the compressed state. Then guided by computational biology results, they identified that a hydrogen bonding and salt bridge interaction network is crucial for the regulation of the conformational dynamics of ClpP. This network, which is composed by residue R171 and D170 from opposing subunits, can stabilize the extended state.
Taken together, this study reveals the dynamic regulation mechanisms of ClpP: substrate peptide enters into ClpP chamber for degradation by catalytic triads including S98, H123 and D172. H123 forms an extensive network of hydrogen bonding with substrate, S98 and D172. After catalytic cleavage of the peptide bond, the accumulation of digested segments in the active site may induce the rearrangement of the hydrogen-bonding network nearby. As a result, H123 shifts away from the active position and is gradually flipped to another orientation, leading to shifting of D172 and the destruction of the R171_D170 contact network. Consequently that extended state can not be stabilized and gradually transforms to compressed state. The peptide-binding pocket disappears with it, leading to the release of products.
On the basis of regulation mechanisms, they have recently identified a few antagonists and inhibitors against ClpP, further researches of computational biology, structural biology and chemical biology are in progress.
This research group combined combine theoretical with experimental approaches to explore the complicated questions in resistant pathogen-associated infections. They also provided critical information for developing novel anti-bacteria drug based on new targets, novel mechanisms and compound structures. The study entitled “Structural switching of Staphylococcus Aureus ClpP: a key to understanding protease dynamics” published in Journal of Biological Chemistry online on September 7th 2011.
This work was supported by grants from the Chinese Academy of Science, National Natural Science Foundation of China, Ministry of Science and Technology of China, and Shanghai Commission of Science and Technology.

