 |
| Apply nickel(II) complex to asymmetric synthesis of chiral amino acids(Image by SIMM) |
Nonproteingenic α-, or β-amino acids have attracted tremendous attention, they are widely utilized for biological, biochemical, pharmaceutical, and asymmetric chemical investigations.
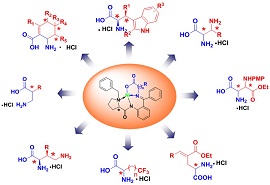
Research group in Shanghai Institute of Materia Medica (SIMM) focuses on the asymmetric synthesis of chiral amino acids via nickel(II) complex (See figure). After several years of focused investigation, they have successfully applied this new methodology to asymmetric Mannich reactions, enantioselective tandem conjugate addition-elimination reactions, asymmetric Michael addition reactions, asymmetric alkylation reactions, Suzuki coupling reactions and asymmetric Diels-Alder reactions.
A series of nonproteingenic amino acids were efficiently obtained including α, β-diamino acids (J. Org. Chem., 2008, 73, 8563-8570), 3-aminoaspartate (Synthesis, 2010, 7, 1205-1208), glutamic acid derivatives (Synthesis, 2009, 10, 1744-1752), β-substituted-α, γ-diaminobutyric acid derivatives (Amino Acids, 2011, 40, in press), linear ω-trifluoromethyl containing amino acids (J. Org. Chem., 2011, 76, 684-687), syn-β-substituted tryptophans (Chem. Commun., 2011, 47 (29), 8355-8357), β2-amino acid derivatives (J. Org. Chem., 2011, 76, 6649-6656), and cyclic amino acid derivatives. These nonproteingenic amino acids provide an efficient arsenal to access diversified bioactive building blocks in drug discovery and development.
In a recent prominent application for heterocyclic amino acids, researchers developed a simple and practical reaction of chiral equivalent of nucleophilic glycine with sulfoyindoles to asymmetric synthesis of syn-β-substituted tryptophan derivaties in high yields and with excellent enantioselectivities. This work has been published online on Chemical Communications (Chem. Commun., 2011, 47 (29), 8355 - 8357). The related thesis was awarded as the ‘Excellent Poster’ at the 7th National Conference on Chemical Biology held in Nanjing on 27th August.
Full text:
http://pubs.acs.org/doi/abs/10.1021/jo8019169
https://www.thieme-connect.com/ejournals/abstract/synthesis/doi/10.1055/s-0029-1219275
https://www.thieme-connect.com/ejournals/abstract/synthesis/doi/10.1055/s-0028-1088075
http://pubs.acs.org/doi/abs/10.1021/jo102031b
http://pubs.acs.org/doi/abs/10.1021/jo200971k
http://pubs.rsc.org/en/Content/ArticleLanding/2011/CC/c1cc12619a
http://www.springerlink.com/content/2p3658632jnj47h4/

