The editing domain (CP1) of leucyl-tRNA synthetase (LeuRS), which was acquired by ancestral LeuRS during evolution, is critical for its catalytic fidelity and subsequently translation accuracy. In addition, CP1 modulates the aminoacylation activity of LeuRS. LeuRSs from various species contain CP1, except LeuRS from Mycoplasma mobile (MmLeuRS). The study on MmLeuRS, a mimic of ancestral LeuRS, could help us understand the evolution of aminoacyl-tRNA synthetase.
TAN Min, YAN Wei and others in the lab of Prof. WANG Enduo at Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Shanghai Institutes for Biological Sciences, CAS, investigated the catalytic and editing characteristic of this unique LeuRS and found that the function of canonical CP1 in aminoacylation reaction could be compensated by a naturally occurring nanopeptide, which replaces the CP1 domain in MmLeuRS. Furthermore, canonical CP1 could substitute the nanopeptide in MmLeuRS and confer it tRNA-dependent editing capability. These results provide a mechanistic framework for the evolution of aaRS by the acquisition of functional modules, and shed a new light on the coordination between the editing and synthetic domain to achieve catalytic efficiency and specificity.
This work entitled “A naturally occurring nonapeptide functionally compensates the CP1 domain of leucyl-tRNA synthetase to modulate aminoacylation activity” was published online in Biochemical Journal on February 1st , 2012, before its appearance in print.
This study was supported by grants from the Chinese Academy of Sciences, the Ministry of Science and Technology, National Natural Science Foundation of China, and the Science and Technology Commission of Shanghai Municipality.
AUTHOR CONTACT:
WANG Enduo
Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
Shanghai, China
Phone: 86-21-54921241; E-mail: edwang@sibs.ac.cn
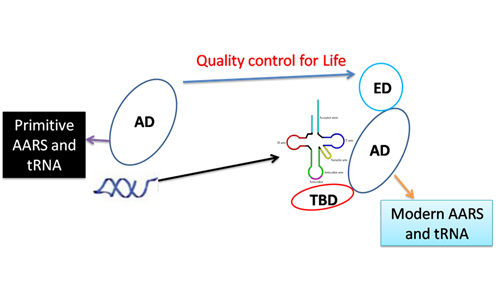
Model for co-evolution of aminoacyl-tRNA synthetase and tRNA
(Image provided by Prof. WANG Enduo)

