Photochromic materials have real or potential applications in protection, display, high-density memory, switch, and many other high-tech areas. A current focus of study in this area is to exploit new materials with better photochromic performances or various switching functions, such as luminescence, electric conductivity, magnetism, and catalysis.
The research group headed by Prof. GUO Guocong at Fujian Institute of Research on the Structure of Matter (FJIRSM) has developed a series of new photochromic materials.
1) Reporting a new room-temperature X-ray-induced photochromic metal complex, and revealing its new X-ray-induced ligand-to-ligand charge-transfer mechanism Angew. Chem. Int. Ed., 2012, 51, 3432–3435, Figure 1). Such species can server as ideal X-ray detection materials, because they are recyclable and also X-rays can be “viewed” directly in color by means of them.
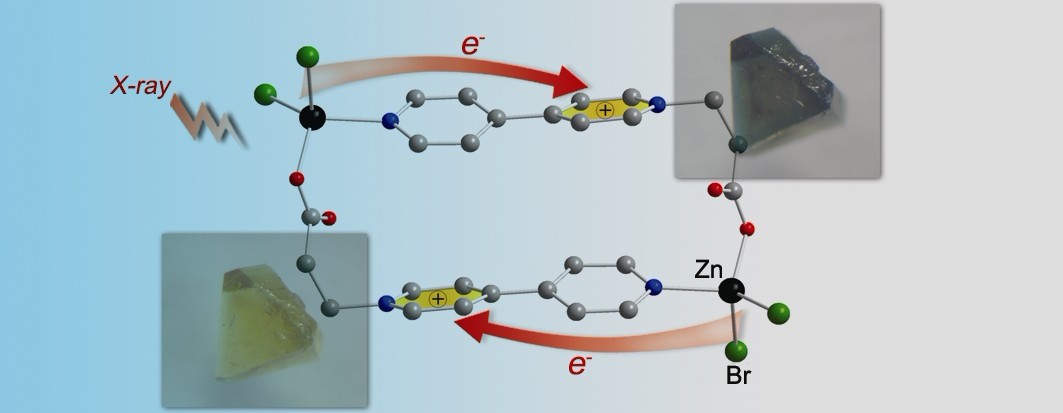
Figure 1: X-ray-induced photochromism of a metal complex with a molecular structure
2) Discovering the redox photochromic behavior of N-methyl-4,4’-bipyridinium (MQ+) salts and their metal complexes, which develops the well-known viologen-based redox photochromic system (Inorg. Chem., 2012, 51, 4015−4019, Figure 2).
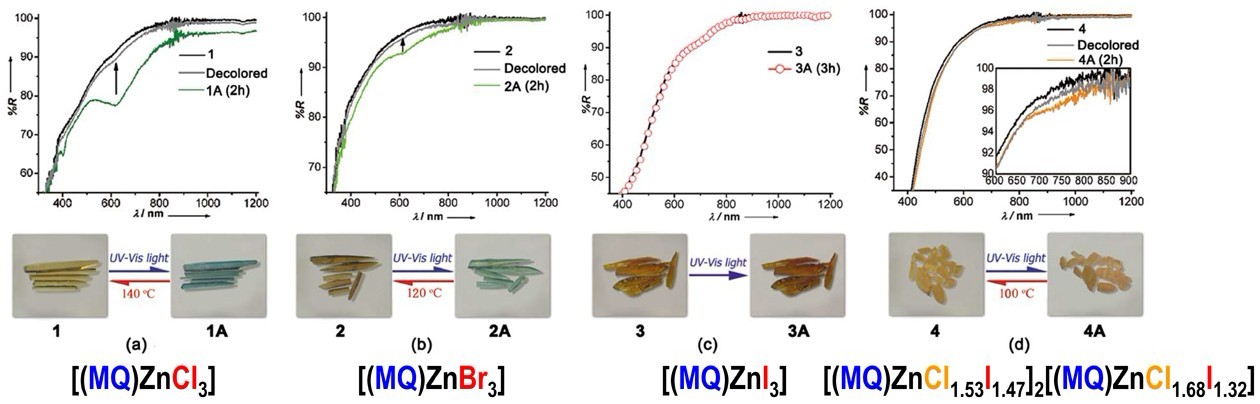
3) Developing new photochromic hybrids of metal halides using the viologen and N-alkyl-aniline analogue cations (Angew. Chem. Int. Ed., 2007, 46, 3249–3251, Figure 3; Angew. Chem. Int. Ed., 2008, 47, 4149–4152), and observing their switchable solid-state photoluminescence and interesting partial photochromic phenomenon (Dalton Trans., 2010, 39, 8688–8692).
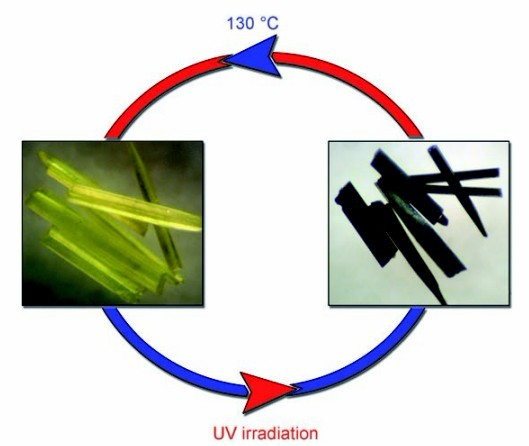
Figure 3: Photochromism of a bismuth(III) chloride-viologen hybrid
4) Opening up a new type of redox photochromic metal complex without photochromic precursors (Angew. Chem. Int. Ed., 2007, 46, 3909–3911, Figure 4), based on a new ligand-based electron-transfer mechanism (Chem. Commun., 2010, 46, 361–376, Feature Article).


