Dr. JIANG Lubin’s group in the Institut Pasteur of Shanghai, Chinese Academy of Sciences (IPS-CAS) has recently discovered an immune evasion mechanism in the severe malaria parasite, which may also be applied to the development of a malaria vaccine. “The implications of these results for our understanding of gene regulation in general and the development of malaria vaccines” were further commented in the “Forum of Malaria” by Dr. Jerry Workman from the Stowers Institute for Medical Research in US and Dr. Mats Wahlgren from the Karolinska Institutet in Sweden in Nature on July 3, 2013.
Malaria is a major tropical parasitic disease in humans. The most deadly form of the human malaria parasite, Plasmodium falciparum, causes more than 1 million deaths and 300-500 million clinical cases worldwide each year. At present, no effective malaria vaccines are available. Thus, development of innovative malaria vaccines is now urgently needed as a top world health priority.
Each P. falciparum encodes 60 antigenically distinct var genes encoding the major virulence factor PfEMP1s on the surface of parasite infected red blood cells. During the blood stage infection, however the clonal parasite population expresses only one gene at a time before switching to the expression of a new variant antigen as an immune evasion mechanism to avoid the host’s antibody responses. The mechanism by which 59 of the 60 var genes are silenced remains largely unknown.
Dr. JIANG Lubin’s group from IPS-CAS has recently identified that a parasite ortholog (PfSETvs) of a fly histone lysine methyltransferase ASH1 controls the epigenetic regulation of the mutually exclusive expression of the var gene family by functioning on histone H3K36me3 at the promoter regions of the var genes, in the worldwide collaborations with other research groups from the NIH, Institut Pasteur, Tongji University, Fudan University and University of Copenhagen, etc.
This study uncovered a novel role of the H3K36me3 mark in eukaryotic gene silencing. In addition, the resulting PfSETvs-knockout parasites expressing all PfEMP1s may also be applied to the development of a malaria vaccine.
The result was published online in Nature on 3rd July and supported by National Natural Science Foundation of China (81271863), the Key Research Program of the Chinese Academy of Sciences (KSZD-EW-Z-003-1-2), the Intramural Research Program of the US National Institutes of Health, and an ERC grant PlasmoEscape (250320).
For more details, please find in the paper PfSETvs methylation of histone H3K36 represses virulence genes inPlasmodium falciparum
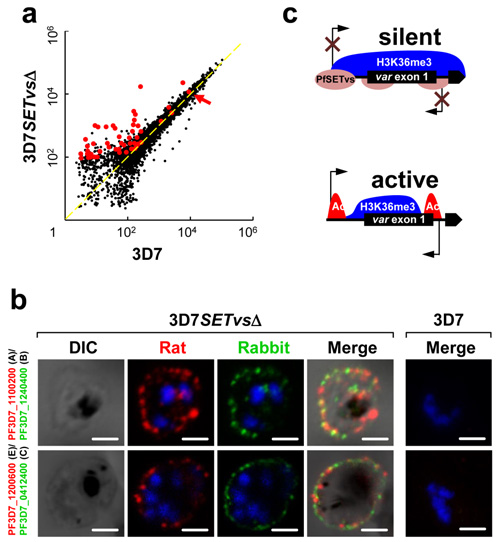
Figure a、b, Knockout of the PfSETvs gene resulted in the transcription of virtually all var genes (a) and their expression as proteins on the surface of individual infected red blood cells (b).
Figure c, Summary diagram showing that the PfSETvs-dependent H3K36me3 enriched along the entire gene body of silent var genes including the TSS of var genes.
(IPS-CAS)

