Bacteria love to colonize surfaces inside your body, but they have a hard time getting past your rugged, salty skin. Surgeries to implant medical devices often give such bacteria the opportunity needed to gain entry into the body cavity, allowing the implants themselves to act then as an ideal growing surface for biofilms. A group of researchers at the Shanghai Institute of Ceramics in the Chinese Academy of Sciences are looking to combat these dangerous sub-dermal infections by upgrading your new hip or kneecap in a fashion appreciated since ancient times -- adding gold. They describe the results of tests with a new antibacterial material they developed based on gold nanoparticles in the journal Applied Physics Letters, from AIP Publishing.
"Implant-associated infections have become a stubborn issue that often causes surgery failure," said Xuanyong Liu, the team's primary investigator at the Shanghai Institute of Ceramics. Designing implants that can kill bacteria while supporting bone growth, Liu said, is an efficient way to enhance in vivo osteointegration.
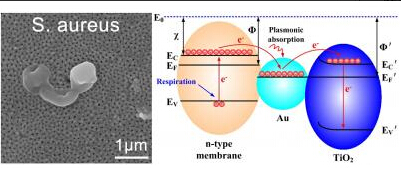
Titanium dioxide is able to kill bacteria itself due to its properties as a photocatalyst. When the metal is exposed to light, it becomes energetically excited by absorbing photons. This generates electron-hole pairs, turning titania into a potent electron acceptor that can destabilize cellular membrane processes by usurping their electron transport chain's terminal acceptor. The membrane is gradually destabilized by this thievery, causing the cell to leak out until it dies.
The dark conditions inside the human body, however, limit the bacteria-killing efficacy of titanium dioxide. Gold nanoparticles, though, can continue to act as anti-bacterial terminal electron acceptors under darkness, due to a phenomenon called localized surface plasmon resonance. Surface plasmons are collective oscillations of electrons that occur at the interface between conductors and dielectrics -- such as between gold and titanium dioxide. The localized electron oscillations at the nanoscale cause the gold nanoparticles to become excited and pass electrons to the titanium dioxide surface, thus allowing the particles to become electron acceptors.
Liu and his team electrochemically anodized titanium to form titanium dioxide nanotube arrays, and then further deposited the arrays with gold nanoparticles in a process called magnetron sputtering. The researchers then allowed Staphylococcus aureus and Escherichia coli to grow separately on the arrays -- both organisms were highly unsuccessful, exhibiting profuse membrane damage and cell leakage.
While silver nanoparticles have been previously explored as an antibacterial agent for in vivo transplants, they cause significant side effects such as cytotoxicity and organ damage, whereas gold is far more chemically stable, and thus more biocompatible.
"The findings may open up new insights for the better designing of noble metal nanoparticles-based antibacterial applications," Liu said.
Further research for Liu and his colleagues includes expanding the scope of experimental bacteria used and evaluating the arrays' in vivo efficacy in bone growth and integration.

