Lung cancer is the leading cause of cancer death worldwide with a 5-year survival rate approximately 15%. Abnormal activation of EGFR caused by EGFR mutations significantly contributes to lung cancer progression and malignancy. In NSCLC samples, EGFR mutations are present in 30-50% of cases in individuals of East Asia descent. By now drugs targeting EGFR have been widely used clinically, such as small-molecule inhibitors gefitinib and erlotinib, which display great inhibitory effect on the kinase activity of EGFR. However, the emergence of drug resistance greatly blocks the function of those inhibitors, while effective countermeasures are still missing. Previous structural and functional studies mainly focused on the EGFR protein itself, while less attention has been paid to the plasma membrane, where EGFR proteins are located. Therefore, exploring the novel mechanisms of EGFR activation is essential to find new strategies to overcome drug resistance.
Under the supervision of Prof. JI Hongbin and Prof. XU Chenqi from the Institute of Biochemistry and Cell Biology (SIBCB), Shanghai Institutes for Biological Sciences and Prof. WANG Hongda from Changchun Institute of Applied Chemistry, WANG Ye and his colleagues identify the distribution pattern of EGFR in the plasma membrane of living cells at single-molecule level with a resolution of ~20 nm using direct stochastic optical reconstruction microscopy (dSTORM).
They find EGFR proteins aggregate to form nanoclusters on the surface of both freshly isolated human lung cancer cells and normal lung epithelial cells. Meanwhile, the number and size of clusters significantly increase in lung cancer cells. They identify the mediation of EGFR clusters formation by anionic phospholipid PIP2 using dSTORM in combination with photoactivated localization microscopy (PALM). Further studies find the ionic protein-lipid interaction between EGFR juxtamembrane (JM) region with positively charged residues and the plasma membrane through binding of PIP2. JM region mutations dramatically block the EGFR clustering. Moreover, disruption of EGFR clusters by PIP2 depletion or JM mutations greatly blocks the activation of EGFR signaling and its biological functions.
Their findings reveal a novel mechanism of EGFR activation from the angle of ionic protein-lipid interaction, and explain the aberrant activation of EGFR in cancers. Their work also provides a hint that disrupting EGFR cluster formation in drug-resistant cancers would be a promising approach to fight with cancers.
This study entitled “Regulation of EGFR nanocluster formation by ionic protein-lipid interaction” was published online in Cell Research on July 8, 2014.
This work was supported by grants from Ministry of Science and Technology of China, the Cross and Cooperation in Science and Technology Innovation Team program, the Strategic Priority Research Program of the CAS, the National Natural Science Foundation of China, and Science and Technology Commission of Shanghai Municipality.
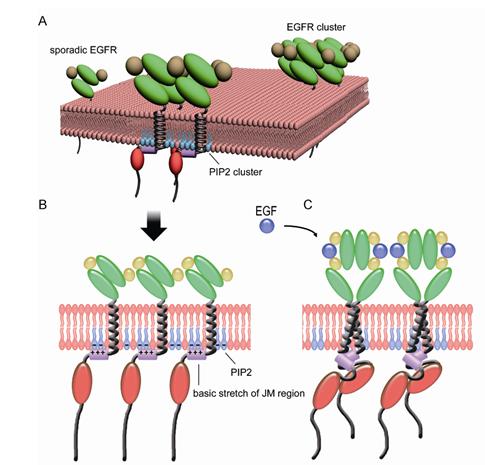
A schematic illustration of ionic protein-lipid interaction-mediated EGFR membrane clustering model (Image by Prof. JI Hongbin's group)

