Stimulator of interferon genes (STING, a.k.a MITA, ERIS or MPYS) is a central component in innate immunity against DNA virus. A research team led by Prof. WANG Chen at the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, demonstrated that the ER adaptor SCAP is essential for STING-mediated signaling and innate antiviral response.
Recent breakthroughs have uncovered several potential DNA-binding proteins to sense the presence of microbial DNAs in the cytoplasm, and consequently to induce the type I interferon production. An emerging theme of the action of these DNA sensors is that, signaling pathways initiating from them are apparently converged on STING, a transmembrane protein in the endoplasmic reticulum (ER). It is recently observed that cytosolic exogenous DNA triggers STING to rapidly dimerize and translocate from ER, through the Golgi apparatus, to the perinuclear microsome compartment. The TANK-binding kinase 1 (TBK1) congregates simultaneously to the same compartment in a STING-dependent manner. Notably, the DNA-driven assembly of STING-TBK1 complex is required for TBK1 activation, which subsequently activates the transcriptional factor IRF3. It remains to be elucidated how IRF3 is recruited onto the STING signalosome.
Under the supervision of Prof. WANG Chen from SIBCB, CHEN Wei, LI Senlin and their colleagues identified SCAP as dynamic and essential component of STING protein complex. Silencing of the ER adaptor SCAP markedly impairs the IRF3-responsive gene expression induced by STING. Scap knockdown mice are more susceptible to HSV-1 infection. Interestingly, SCAP also translocates from ER, via Golgi, to perinuclear microsome. Mechanistically, the N-terminal transmembrane domain of SCAP interacts with STING, and the C-terminal cytosolic domain of SCAP binds to IRF3, thus recruiting IRF3 onto STING signalosome.
This study characterizes SCAP as an essential adaptor in the STING signaling pathway, uncovering a critical missing link in DNAs-triggered host antiviral responses.
This work entitled “ER Adaptor SCAP Translocates and Recruits IRF3 to Perinuclear Microsome Induced by Cytosolic Microbial DNAs” was published online in PLoS Pathogens in February 22, 2016. It was supported by grants from the Ministry of Science and Technology of China, National Natural Science Foundation of China.
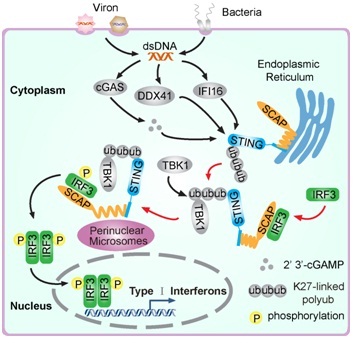
Fig1. Upon microbial DNA sensing, the ER adaptor SCAP translocates and recruits the transcription factor IRF3 onto perinuclear microsomes, thus making the kinase TBK1 could phosphorylate and activate IRF3. Insights gained in this study are highlighted by the colored diagram. (Image by WANG Chen’s Group)

