Sulfur (VI) fluoride compounds are overlooked in history until recently Sharpless’ lab (Angew. Chem. Int. Ed. 2014, 9430) reported the sulfur (VI) fluoride exchange transformations (SuFEx), (Figure 1). Great applications of this type of function groups emerged in material science, chemical biology and synthetic chemistry. The excellent stability of sulfur (VI) fluorine bond is kinetic rather than thermodynamic in origin since it is thermodynamically unstable with respect to water. They are relatively unreactive towards most categories of chemical reactions (hydrolysis, reduction, nucleophilic substitution, thermolysis, etc.). When reactions do occur, they appear to react exclusively at the sulfur center. The contrast between the high kinetic stability of S(VI)-F bond and their activatable sulfur (VI) fluoride exchange (SuFEx) chemical reactivity resulting in fringe acid-base reactivity. These properties designate SuFEx reactivity as a potentially powerful, context-dependent variant of click chemistry.
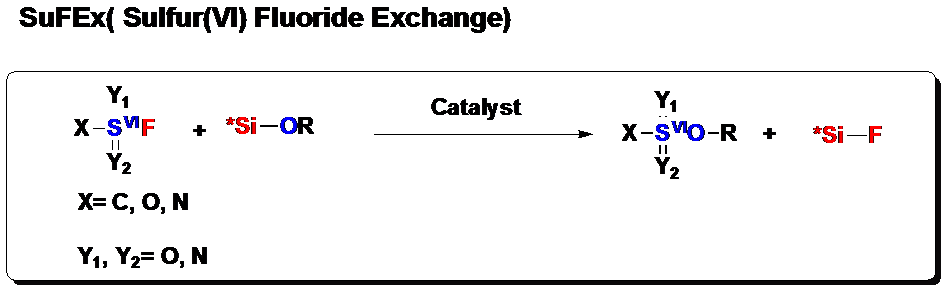
Figure 1. Sulfur(VI) Fluoride Exchange Reaction, (image by Dong).
Collaborating with Prof K. Barry Sharpless Group and Peng Wu in TSRI (The Scripps Research Institute), Dong’s group from Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences has successfully developed Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates in Nature Chemistry.
Polysulfates and polysulfonates with their sulfur(VI) linkages(–SO2–) possess excellent mechanical properties and high chemical stability, as needed for engineering polymers. For instance, the bisphenol A (BPA) polysulfate has the slightly higher tensile modulus, similar yield stress and significantly lower oxygen permeability compared to its polycarbonate counterpart. Nevertheless, they have rarely been used for industrial applications due to a lack of reliable and scalable synthetic access. The SuFEx process represents the first efficient protocol for the preparation of these polymers. Considering the availability of bisphenols (BPA has an annual production of millions of tonnes, most of which are used for polycarbonates and epoxy resins industry) and sulfuryl fluoride gas (SO2F2, widely used as fumigant) as industrial feedstocks, it is attractive to explore this process for potential industrial applications further.
This work developed bifluoride salts as a new class of catalysts for the SuFEx click reaction, and have applied this chemistry to the preparation of polysulfates and polysulfonates. Compared to organosuperbase catalysts (for example, DBU and BEMP), the bifluoride ion [FHF]– is acidic. It allows the SuFEx polymerization reaction to be extended to a broader substrates scope, such as aliphatic sulfonyl fluorides. Most importantly, the bifluoride catalyst is significantly more active. Therefore the polymerization reaction requires much lower catalyst loading, which is essential for cost-effective industrial scale production. This work sets a foundation for future translation of polysulfate synthesis from laboratory research to industrial applications.
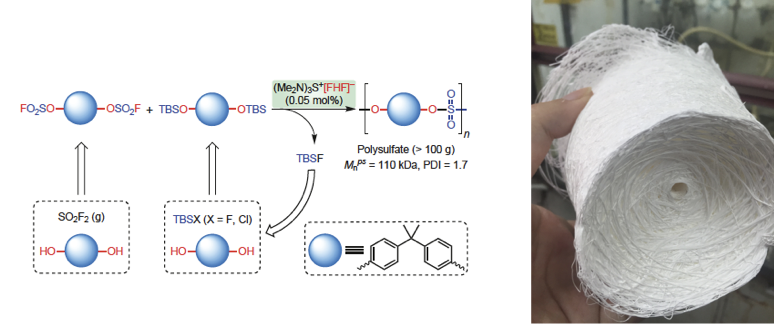
Figure 2. Bifluoride-catalysed SuFEx reaction for the synthesis of BPA-polysulfate (image by Dong)
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, “One Hundred Talents Program” supported by SIOC, National Science Foundation (NSF) of China, and Shanghai Science and Technology Committee (S&TCSM).
Contact:
Jiajia Dong,Ph.D.
Research Professor
Key Laboratory of Organofluorine Chemistry

