Chiral compounds are ubiquitous in the areas of biological science, medicinal chemistry, materials science, etc. The enrichment of the chiral pool is highly related to the progress of novel asymmetric synthetic methods. In this regard, catalytic asymmetric dearomatization (CADA) has emerged as an effecient strategy for the construction of various chiral molecules from readily available planar aromatic compounds (Angew. Chem. Int. Ed. 2012, 51, 12662. Chem 2016, 1, 830.). On the other hand, multiple substituted tetrahydrofurobenzofurans are the core structures of a number of biologically active natural products and pharmaceuticals, including (-)-panacene, (+)-gynunone, etc. Thus, a new method for the rapid construction of these structures is highly desirable.
The YOU group from Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences has focused on CADA reactions for several years. Endowing arenes with novel types of reactivity is a long time pursuit in this group. To expand the range of CADA reactions, a strategy of altering an arene from a nucleophile to an electrophile by decorating an electron-withdrawing group seems to be a good strategy. Recently, the YOU group has successfully developed palladium-catalyzed highly stereoselective dearomative [3+2] cycloaddition of nitrobenzofurans, which has been accepted by Chem (DOI: 10.1016/j.chempr.2017.06.015).
With this method, numerous substituted tetrahydrofurobenzofurans were synthesized directly in good to excellent yields, diastereo- and enantioselectivity. It is worthy to note that nitrobenzothiophenes could also be tolerated as substrates in this reaction to construct tetrahydrofurobenzothiophenes. The wide scope for both substrates and easily transformable functional groups on the products make this methodology potentially useful for synthetic chemistry. In addition, a plausible catalytic cycle involving stereodetermining dearomative addition of the alcohol anion to nitrobenzofuran followed by fast allylic cyclization was proposed. 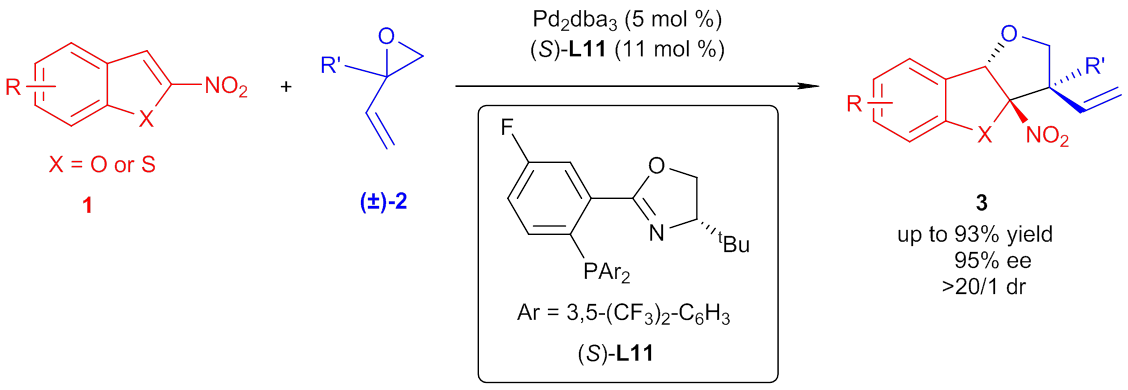
Palladium-catalyzed asymmetric dearomatization of nitrobenzofurans (Image by the YOU group)
Generally, the reported methodology proved to be highly efficient in asymmetric construction of tetrahydrofurobenzofurans and provided a new direction for the strategic development of asymmetric dearomatization processes.
This work was financially supported by National Basic Research Program of China from Ministry of Science and Technology (MOST), National Science Foundation of China (NSFC), the Strategic Priority Research Program of the Chinese Academy of Sciences. Contact Author: Shu-Li You Shanghai Institute of Organic Chemistry, CAS E-mail: slyou@sioc.ac.cn |

