Attachment of poly-ubiquitin (poly-Ub) chains to a protein is a class of versatile posttranslational modifications and plays critical roles in numerous biological processes and diseases. As a unique type of poly-Ub chain, the linear (also called the M1-linked) poly-Ub chain, is widely involved in immune signaling pathways. HOIP is the only one currently known E3 ligase capable of catalyzing linear Ub chain formation, and forms a functional complex with SHARPIN and HOIL-1L called the linear Ub chain assembly complex (LUBAC). Importantly, malfunction of any of the three LUBAC subunits causes inflammation, immunodeficiency or even death in animal models and/or human. Interestingly, the isolated HOIP has low activity due to the auto-inhibition between its UBA domain and catalytic core. Currently, it has been well demonstrated that the both HOIL-1L and SHARPIN can activate HOIP by direct binding, but how the UBA domain of HOIP inhibits the catalytic core remains elusive. Therefore, the molecular mechanism for the activation of HOIP by SHARPIN or HOIL-1L remains to be elucidated.
In a study from the State Key Laboratory of Bioorganic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, researchers solved the crystal structure of SHARPIN UBL in complex with HOIP UBA domain and uncover the molecular mechanism for the specific interaction between SHARPIN and HOIP. They further demonstrate that SHARPIN and HOIL-1L can separately or synergistically bind to different sites of HOIP UBA domain with induced allosteric effects, thereby likely causing a conformational re-arrangement between UBA and the catalytic core region to facilitate the E2 loading of HOIP for the subsequent linear Ub chain assembly. This study is recently published in the journal Cell Reports with a title “Structural Insights into SHARPIN-Mediated Activation of HOIP for the Linear Ubiquitin Chain Assembly” (Cell Reports, October 3, 2017, 21, 1–10).
Although the interaction between HOIL-1L and HOIP has been clearly demonstrated that the UBA domain of HOIP directly binds to the UBL domain HOIL-1L, the molecular mechanism for the interaction between HOIP and SHARPIN is still elusive. Particularly, which domain (the UBA or the NZF2 domain) of HOIP binds to SHARPIN remains controversial in previous studies. This study clearly elucidates that SHARPIN also binds to the UBA domain, but in a manner distinct from the HOIL-1L binding, thus revealing the assembling mechanism of the LUBAC complex.
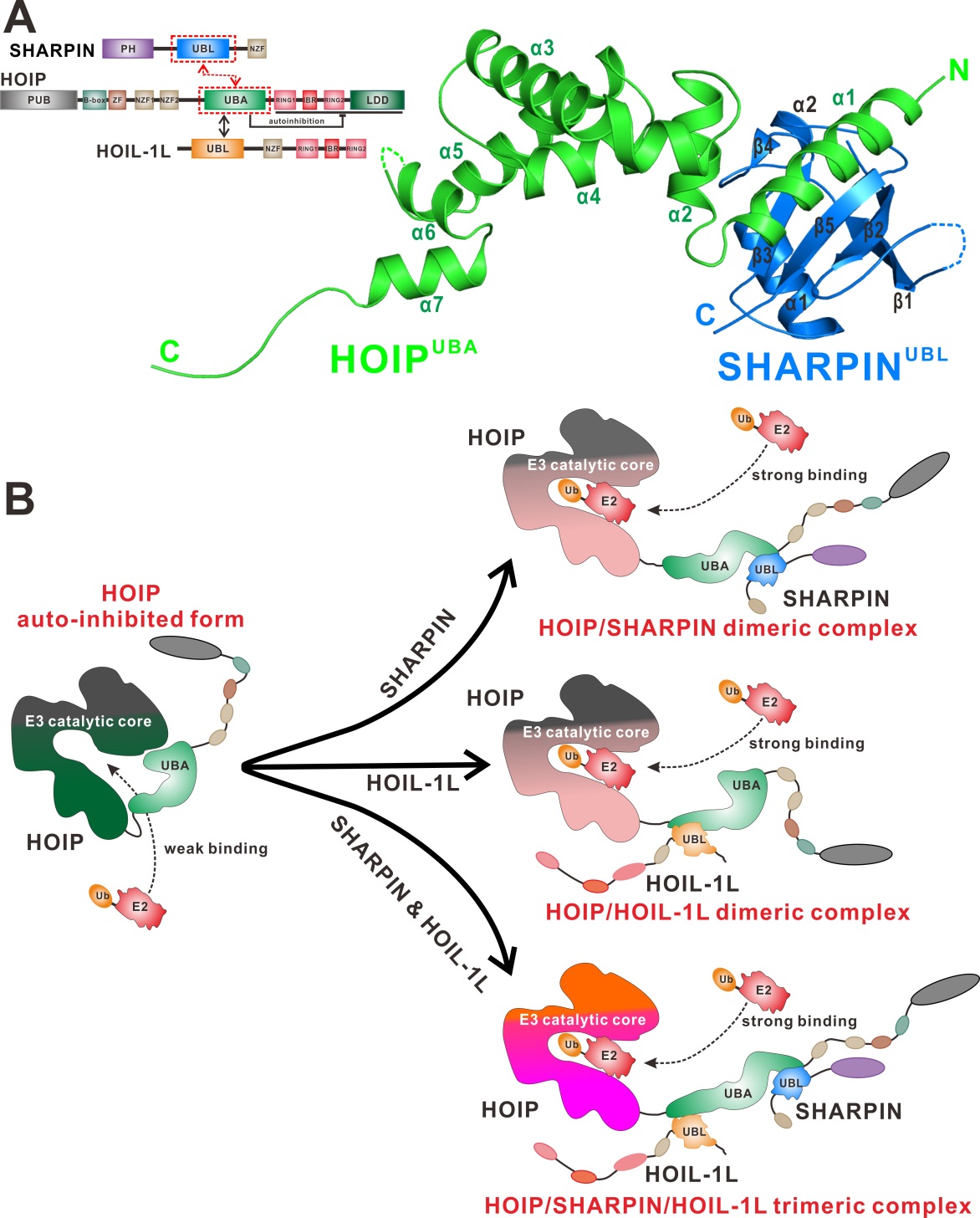
Mechanism of SHARPIN and/or HOIL-1L meditated activation of HOIP for linear ubiquitin chain assembly. (A) The domain organizations of SHARPIN, HOIP and HOIL-1L proteins as well as the crystal structure of HOIP/SHARPIN complex. (B) Both SHARPIN and HOIL-1L binds to HOIP UBA domain and facilitates E2 loading to the catalytic core of HOIP. (Image by Jianping Liu)
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Ministry of Science and Technology of China, “1000-Youth Talents Plan”, National Science Foundation of China (NSFC), and Shanghai Science and Technology Committee (STCSM).

