On March 28th, 2018, Nature Communications published a research work entitled “MLL5 suppresses antiviral innate immune response by facilitating STUB1-mediated RIG-I degradation" from Yan Zhang’s unit, Institute Pasteur of Shanghai, Chinese Academy of Sciences. This work demonstrates that MLL5, an epigenetic regulator, negatively regulates antiviral innate immune responses through directly facilitating the interaction between RIG-I and its E3 ligase STUB1 in the cytoplasm, thereby promoting STUB1-mediated degradation of RIG-I.
RIG-I can recognize cytosolic viral RNA and induce production of type I IFNs and proinflammatory cytokines. Inappropriate production of inflammatory cytokines leads to immune toxicity, therefore, the degradation of RIG-I illustrates an important mechanism for normal immune responses and immune homeostasis. Trithorax family proteins are important epigenetic regulators that control genes expression through modulating chromatin structure and deposition of activating H3K4 methylation marks on histones. MLL5 is an important epigenetic modifier that controls cell cycle progression, chromatin architecture maintenance and hematopoiesis. However, whether MLL5 has a role in innate antiviral immunity is largely unknown.
Under the guidance of Prof. Yan Zhang, Ph.D candidate Peipei Zhou, on the basis of the master work (THE JOURNAL OF BIOLOGICAL CHEMISTRY,2013:288:17532-17543), further investigated that MLL5 suppresses the RIG-I-mediated anti-viral immune response. Mll5-deficient mice infected with vesicular stomatitis virus show enhanced anti-viral innate immunity, reduced morbidity and viral load. Mechanistically, a fraction of MLL5 located in the cytoplasm interacted with both RIG-I and its E3 ubiquitin ligase STUB1, which promotes K48-linked polyubiquitination and proteasomal degradation of RIG-I. MLL5 deficiency attenuated the RIG-I and STUB1 association, reducing K48-linked polyubiquitination and accumulation of RIG-I protein in cells. Upon virus infection, nuclear MLL5 protein translocated from the nucleus to the cytoplasm inducing STUB1-mediated degradation of RIG-I.
This work was kindly supported by Guangxun Meng (Institut Pasteur of Shanghai), Hui Xiao (Institut Pasteur of Shanghai), Jin Zhong (Institut Pasteur of Shanghai), Fajian Hou (Institute of bicochemistry and cell biology), Bin li (Shanghai Institute of Immunology, Shanghai Jiaotong University), and Lihwen Deng (National University of Singapore). And this study gets financial supported from the National Basic Research Program of China, the National Natural Science Foundation of China and the Chinese Academy of Sciences.
Full text link: https://www.nature.com/articles/s41467-018-03563-8
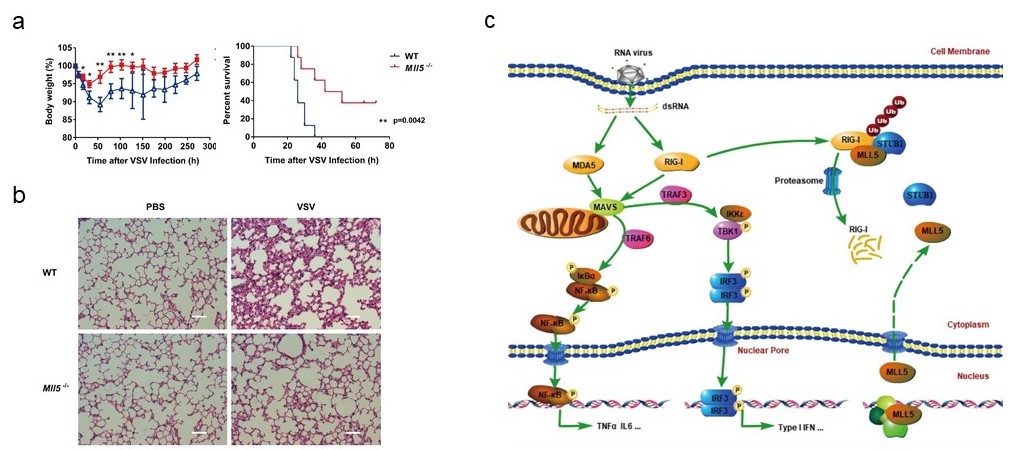
(a) The body weight and mortality curve of WT and Mll5-/- mice infected with VSV-GFP. (b) Hematoxylin-eosin staining of the lung sections from WT and Mll5-/- mice infected with VSV-GFP. Scale bar, 200 μm. (c) Working model. |

