Natural gas and carbon dioxide can be converted into synthesis gas and then further into various important chemicals, which can efficiently utilize natural gas and effectively reduce greenhouse gas emissions. However, the problem of carbon deposition and catalyst sintering/agglomeration at high temperatures which results in the attenuation of conventional reforming performance remains unsolved.
Prof. XIE Kui’s Group at Fujian Institute of Research on the Structure of Matter of Chinese Academy of Sciences designed the solid oxide electrolysis cell combining the two gas-phase electrochemical conversion processes of carbon dioxide electrolysis (CO2+2e-=CO+O2-) and methane oxidation (CH4+O2-=CO+2H2+2e-). The study was published online in Science Advances.
This group has established a new system of composite eletrode surface interfaces by synergistically controlling the non-stoichiometric ratio of ceramic electrodes and doping, which greatly improves the performance of direct cathode electrolysis of CO2.
In this study, the researchers realized the new process of catalyzing methane/carbon dioxide synthesis, and clarified the reforming mechanism of CH4/CO2.
To enhance electrode durability, exsolved metal-oxide interfaces were in situ grown on ceramic electrode scaffold by synergistic control of non-stoichiometry and doping. This in- situ formed interfaces are favorable for CO2 activation and coking resistance. And the electrochemically-pumped oxygen species are highly beneficial to the carbon removal at interfaces even in the presence of H2 or H2O atmosphere.
By in-situ engineering the interface architecture and composition, a high performance electrochemical reforming process was demonstrated. The atom efficiency and current efficiency for the electrochemical reforming of CH4/CO2 are up to 100%. And the in situ exsolved metal/oxide interface provides optimal stability with no degradation being observed even after 300 hours of high temperature operation and 10 redox cycles.
This is the first demonstration of a highly efficient electrochemical reforming of CH4/CO2 to produce syngas in a solid oxide electrolyser with remarkably enhanced coking resistance and catalyst stability.
The study was supported by the National Fund's major research program “Catalytic Science of Carbon-Based Energy Conversion and Utilization” and the “Hundred Talents Program” of Fujian's Pioneering Innovative Talents.
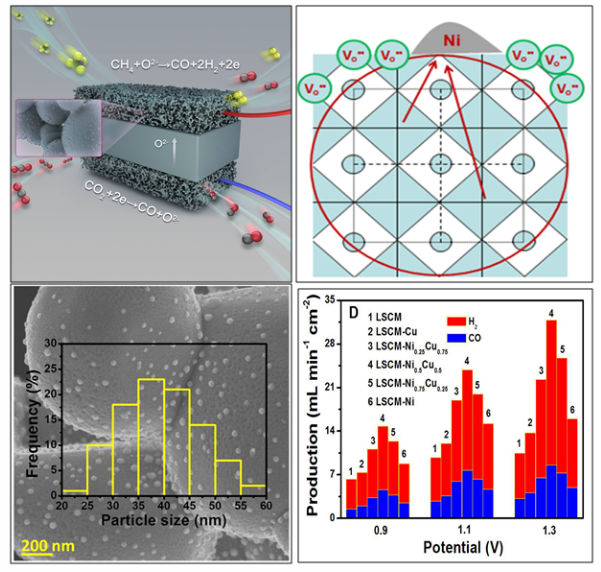
Schematic representation of electrochemical CO2/CH4 reforming process in a solid oxide electrolyser to produce syngas(Image by Prof. XIE’s group).
Contact:
Prof. XIE Kui
Fujian Institute of Research on the Structure of Matter
Chinese Academy of Sciences
Email: kxie@fjirsm.ac.cn

