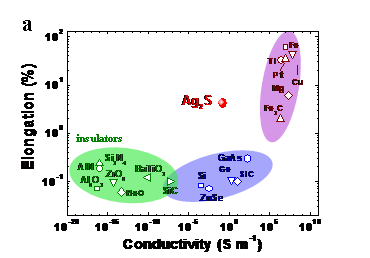
Ductility is characteristic phenomenological feature of metals and metallic materials. For the semiconductors and insulators, it is rarely observed. The scientists from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences and Max-Planck-Institut für Chemische Physik fester Stoffe in Dresden reported the extraordinary metal-like ductility with high plastic deformation strainsat room temperature for the semiconductor a-Ag2S, which is used in a variety of electronic and optoelectronic devices. This is one of the results of long-term collaboration between the groups of Profs. Lidong Chen, Xun Shi, and Yuri Grin. The study is published in the latest issue of Nature Materials.
Differently to majority of semiconductors and ceramics being usually brittle, however, the inorganic semiconductor α-Ag2S is ductile like a metal and easily changes shape instead of breaking under outer deformation. The scientists adopted castingand Spark Plasma Sintering techniques to obtain bulk α-Ag2Ssamples, which can be easily machined to cylinder or bar shapes.
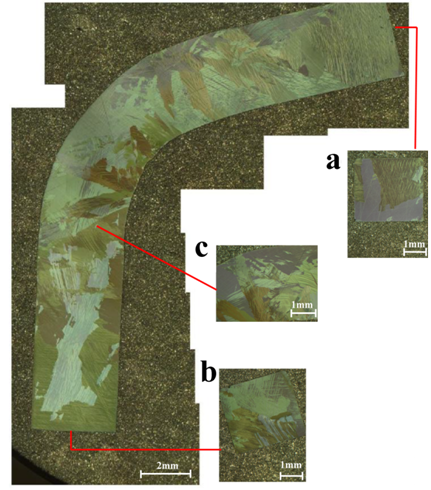
The so-obtained laths are very thin and wind wire-like. The cylindrical ingot of α-Ag2S can be easily hammered or uniaxial deformed to a very large grade without destroying the material (Figure left).
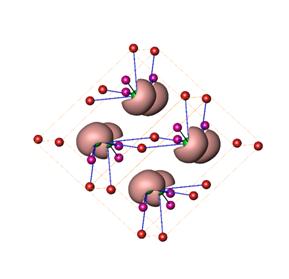
The scientists observed the unified picture bycombining the results of different manufacturing techniques and physical characterization with the quantum chemical calculations and analysis of atomic interactions. Furthermore, the unusual behavior of the silver sulfide appears due to the preventing of cleavage on the slip planes within the crystal structure by the irregularly distributed bonds of silver atoms (across) these planes.
The scientists analysed the chemical bonding by using quantum chemical techniques in position space (QTAIM, ELI), and revealed a moderate charge transfer from silver to sulphur and formation of lone-pairs on sulphur atoms. Therefore, the latter are head-to-head arranged along two types of the crystallographic planes within the crystal structure with relatively atomic weak interactions.
This feature allows the slipping of the structural units along these planes and – in case of ionic materials – causes cleavage. Moreover, the latter is prevented by the irregular across-plane bonds formed by a pert of silver atoms which move (diffuse) within the structure (Figure right).

Contact:
Xun SHI
Shanghai Institute of Ceramics
E-mail:xshi@mail.sic.ac.cn
Reference:
Room-temperature ductile inorganic semiconductor
https://www.nature.com/articles/s41563-018-0047-z

