Direct conversion of methane to chemicals at mild conditions is regarded as the “Holy Grail” reaction in catalysis science, which has attracted numerous research interests from both industrial and academics. Compared with traditional C1 products including CH3OH and HCOOH, conversion of methane to C2 products involving C-C coupling is more economic and challenging. In the previous studies for methane conversion to C2 products, the low catalytic activity and selectivity with the usage of noble metals are still far behind the industrial application.
Inspired by these challenges, a research team led by Prof. Yuhan Sun and Prof. Liangshu Zhong at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported the direct conversion of methane to acetic acid (CH3COOH) with ultrahigh selectivity in oxygenated products by direct coupling of CH4, CO and H2O2 over ZSM-5 supported Fe binuclear sites under 30 °C. The research results were published in Chem entitled "Fe binuclear sites convert methane to acetic acid with ultrahigh selectivity”.
The ZSM-5 supported Fe binuclear sites can afford 89% total oxygenates selectivity with 66% being CH3COOH in oxygenated products at 30 °C. Particularly, 100% CH3COOH selectivity in oxygenated products can be obtained over Fe-BN/ZSM-5 by optimizing reaction conditions if the formed CO2 was not taken into consideration.
The unexpected ultrahigh selectivity towards CH3COOH was attributed to the unique Fe binuclear sites structure of [Fe(III)-(μO)2-Fe(III)-(OH)2] , which was evidenced by advanced spectroscopic techniques and density functional theory (DFT) calculations.
The unique structure of [Fe(III)-(μO)2-Fe(III)-(OH)2] also promoted the formation of CH3COOH by direct coupling of ·CH3 with CO* and OH*, compared with the oxidation of CH4 by OH* to form CH3OH, benefited the CH3COOH formation.
Large usage of precious metal catalysts, low oxygenates (sole product) yield and selectivity are the main challenges for methane direct conversion. The findings in this work may provide an efficient pathway and low-price catalysts for direct conversion of CH4 to CH3COOH with high yield and selectivity.
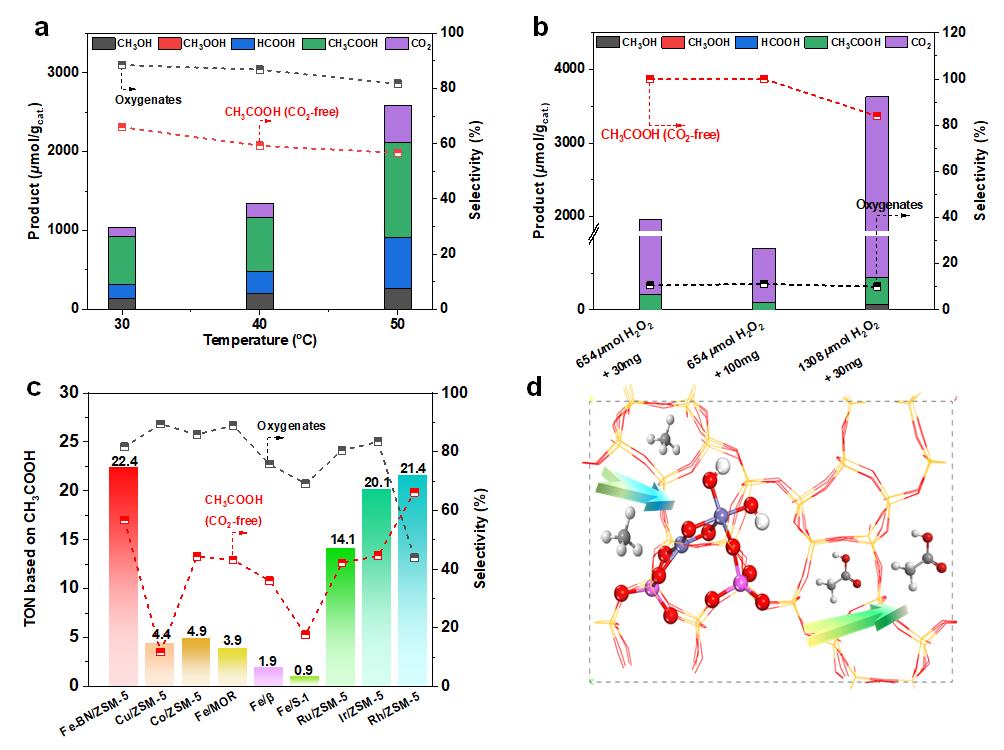
Catalytic performance and schematic illustration for methane conversion to acetic acid (Image by SARI)
Contact: ZHONG Liangshu
Shanghai Advanced Research Institute
zhongls@sari.ac.cn

