Recently, the team of Atmospheric Environment led by Professor CHEN Jinsheng in the Institute of Urban Environment, Chinese Academy of Sciences (IUE, CAS) explored the atmospheric peroxyacetyl nitrate pollution mechanism and its impact on ozone in a coastal city of Southeast China with an Observation-Based Model coupled to the Master Chemical Mechanism.
Peroxyacetyl nitrate (PAN) is a key product of photochemical smog. PAN is generated through photochemical reactions of precursors emitted by human activities only, and the atmospheric PAN is a reliable and scientific indicator of photochemical pollution. In the surface atmosphere, the level of PAN is much lower than that of ozone (O3), but its biological toxicity is about one or two magnitudes greater than that of O3. Additionally, PAN acts as a temporary reservoir for NOx and radicals, and can transport to remote regions to redistribute NOx and intervene in O3 production at regional or even global scale.
The intensive observation campaigns were conducted in spring and autumn to reveal the pollution characteristics of PAN and its impact on O3, and the contributions of influencing factors to PAN formation were also quantified in this paper. The F-values of generalized additive model (GAM) results reflecting the importance of the influencing factors showed that ultraviolet radiation (UV, F=60.64), Ox (Ox=NO2+O3, F=57.65), and air temperature (T, F=17.55) were the main contributors in the PAN pollution in spring, while the significant effects of Ox (58.45), total VOCs (TVOCs, 21.63) and T (20.46) were found in autumn. The PAN formation rate in autumn was 1.58 times higher than that in spring, relating to the intense photochemical reaction and meteorological conditions. Model simulations revealed that acetaldehyde oxidation (46±4%) contributed to the dominant formation pathway of PA (hence PAN), followed by methylglyoxal oxidation (28±3%) and radical cycling (19±3%). The PAN formation was highly VOC-sensitive, as surplus NOx (compared with VOCs abundance) prevented NOx from being the limiting factor photochemical formation of secondary pollution. At our site, PAN promoted and inhibited O3 formation under high and low ROx levels, respectively. The PAN promoting O3 formation mainly occurred during the periods of 11:00-16:00 when the favorable meteorological conditions (high UV and T) stimulated the photochemical reactions to offer ROx radicals, which accounted for 17% of the whole monitoring periods in spring and 31% in autumn. The analysis of PAN formation mechanism and its positive or negative effect on ozone provided scientific insights into photochemical pollution mechanism under various pollution scenarios in coastal areas.
The research results were published on Atmospheric Chemistry and Physics under the title of Seasonal characteristics of atmospheric peroxyacetyl nitrate (PAN) in a coastal city of Southeast China: Explanatory factors and photochemical effects. PhD student LIU Taotao from the Institute of Urban Environment is the first author. Professor CHEN Jinsheng and Associated Professor HONG youwei from the Institute of Urban Environment and Professor are the corresponding authors. This study was funded by the Cultivating Project of Strategic Priority Research Program of the Chinese Academy of Sciences (XDPB1903), the foreign cooperation project of Fujian Province (2020I0038) and Xiamen Atmospheric Environment Observation and Research Station of Fujian Province.
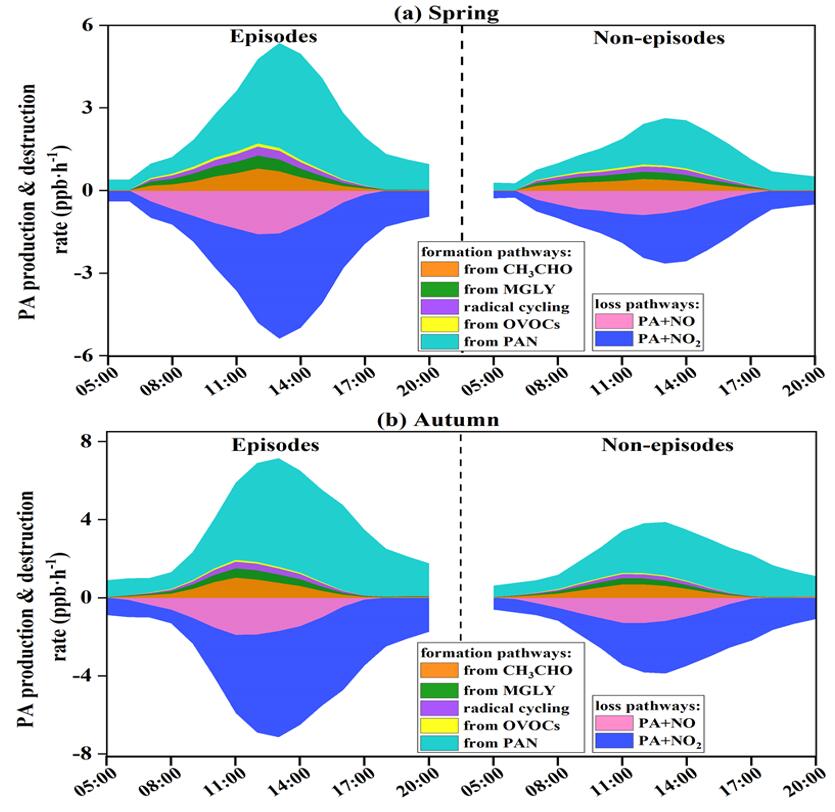
Figure 1. Formation and destruction rates of PA radical (hence PAN) during episodes and non-episodes in (a) spring and (b) autumn, respectively.

Figure 2. Model-simulated O3 budgets and OH, HO2, and RO2 on the inhibition effect stages and promotion effect stages.

