A recent study published in Journal of Experimental Medicine demonstrated that astrocytic dopamine receptor D2 (DRD2) plays a key role in the pathogenesis of multiple sclerosis (MS). This was a collaboration between research groups led by Professor ZHOU Jiawei from the Institute of Neuroscience, Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences and Professor LIANG Xinmiao from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
MS is an immune-mediated disease in the central nervous system (CNS), which is characterized by chronic and progressive inflammation, demyelination and neurodegeneration. Many patients are at first diagnosed with relapsing–remitting form of the disease which likely progresses into a second phase, secondary progressive multiple sclerosis (SPMS). Although there are more than a dozen of disease-modifying drugs available for MS treatment, the efficacy of these treatments remains limited. Emerging evidence has suggested that the deregulated CNS innate immunity plays crucial roles on the onset and progression of the disease. Understanding on this highly complex process remains very limited. Therefore, it is critical to identify and characterize the cellular and molecular basis of disrupted CNS innate immunity in MS that provides a new avenue for the intervention of the disease progression.
Despite the increasing appreciation of the importance of CNS glial cells under pathological conditions, including experimental autoimmune encephalomyelitis (EAE), an animal model of MS, and MS, the molecular and cellular mechanisms underlying the regulation of astrocyte-associated CNS innate immunity and the functional contribution of these mechanisms to the EAE/MS pathogenesis remain elusive. In this study, they identify DRD2 in astrocytes (aDRD2), but not in neurons, as a determinant of white matter astrocyte activation leading to the sustained inflammation primarily within the CNS during EAE. They found that selective ablation of aDrd2 remarkably abrogated EAE progression. Moreover, aDrd2 tightly controlled the expression of PTS which robustly regulates NF-κB pathway-dependent inflammatory response via PKC-delta. Treatment of either dopamine or DRD2 agonist cabergoline activated NF-κB pathway. Blockade of aDRD2 signaling using DHCB, a DRD2 inhibitor derived from herb Corydalis, remarkably attenuated EAE phenotype. These data suggest that DRD2/PTS/PKCd signaling in astrocytes plays a key role in the propagation of inflammatory response that is a critical machinery driving neurodegeneration in EAE.
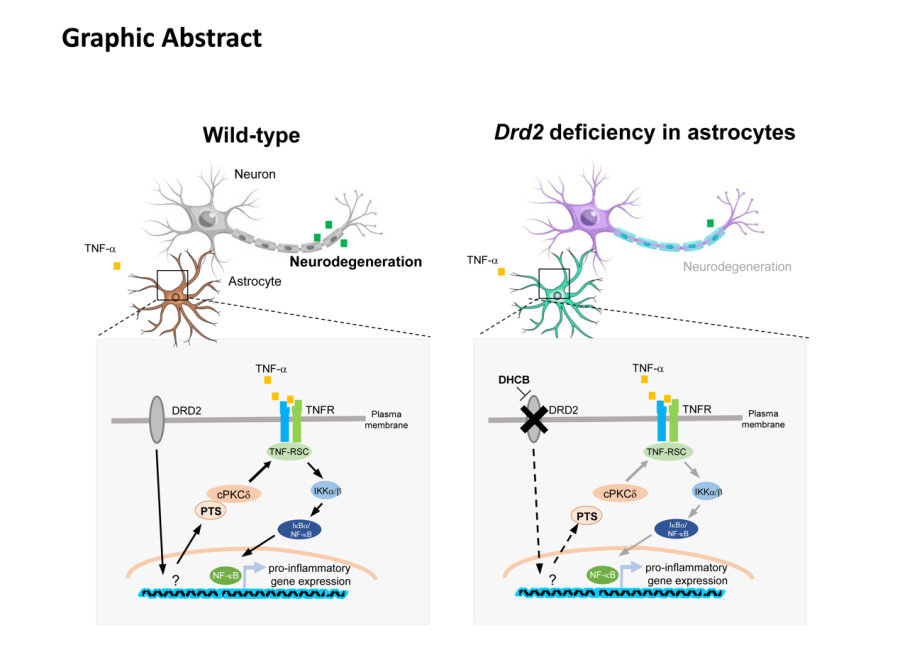
Figure legend: Astrocytic DRD2 promotes neuroinflammatory responses in EAE
In summary, this study reveals previously uncharted mechanisms of disease pathogenesis and raises the possibility that blockade of DRD2/PTS signaling in astrocytes could provide therapeutic benefit in autoimmunity diseases such as MS.
This work entitled “Inhibition of astrocytic DRD2 suppresses CNS inflammation in an animal model of multiple sclerosis” was published online in Journal of Experimental Medicine on July 26, 2022. LU Shenzhao, WU Yue, GUO Yongshun, LIANG Peizhou and YIN Shu are the co-first authors of this paper. This work was supported by the Chinese Academy of Sciences, Shanghai Municipality, the Ministry of science and technology and National natural science foundation of China.
Keywords: multiple sclerosis, astrocytes, DRD2
AUTHOR CONTACT:
ZHOU Jiawei
Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China.
E-mail: jwzhou@ion.ac.cn

