Recently, researchers led by Prof. HU Guohong from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences (CAS) and collaborators, devoted to the studies of cancer metastasis, revealed a new mechanism of lung cancer bone metastasis. Entitled “IL20RB mediates tumoral response to osteoclastic niches and promotes bone metastasis of lung cancer”, these findings were published in Journal of Clinical Investigation on August 25th.
Bone is the most frequent target site of distant metastasis for cancers including breast cancer, lung cancer and prostate cancer. Bone metastasis usually causes fracture, pain or even death of patients. Due to the special structure of the osseous tissue, the regulation of tumor metastasis to bone is clearly different from that to other soft tissues.
Numerous studies have focused on cancer bone metastasis. Present comprehension of osteolytic bone metastasis considers that there is a “vicious cycle” between tumor cells and osteoclasts. Cytokines from tumor cells promotes the maturation of osteoclasts from mononuclear precursors; in turn, osteoclasts digest bone matrix, leading to the release of embedded growth factors, such as transforming growth factor β (TGF-β), insulin-like growth factor (IGF), and calcium to facilitate tumor cell seeding and proliferation.
However, the involvement of osteoclasts in bone metastasis is far from being completely understood. Other than bone resorption, it was not clear whether osteoclasts could act directly on tumor cells.
Prof. HU Guohong’s research group and collaborators found that osteoclast-derived IL19 could directly regulate the proliferation of lung cancer cells in bone through tumor-expressed IL20RB.
Researchers firstly analyzed the expression data of clinical samples and found that the expression of IL20RB was positively correlated with lung cancer bone metastasis. Next, by the in vitro and in vivo assays, they demonstrated that IL20RB could indeed promote bone metastasis. Further studies showed that IL20RB mediated tumor cell responses to pro-tumor signals directly sent by osteoclasts. Tumor cells induced osteoclasts to secrete IL19, the ligand of IL20RB; IL19 bound to IL20RB on the surface of tumor cells and activated downstream JAK1-STAT3 signaling, thereby enhancing the proliferation of tumor cells in bone. However, lung cancer cells that did not express IL20RB, or when IL20RB and IL19 were inhibited, would not respond to the pro-tumor signals. In addition, the researchers also developed a neutralizing antibody against IL20RB, which could effectively inhibit the binding of IL19 to IL20RB and had an obvious therapeutic effect on bone metastasis of lung cancer in vivo.
This study elucidated a mechanism of direct osteoclast-to-tumor stimulus for lung cancer bone metastasis and supported therapeutic targeting of tumoral responses to osteolytic niches, in addition to the current anti-osteoclast therapeutics, for metastasis treatment.
HE Yunfei from Prof. HU Guohong’ s group is the first author of this article. Prof. HU Guohong from SINH, Dr. YAO Feng from Shanghai Chest Hospital and Dr. FU Da from Shanghai Tenth People’ s Hospital are the co-corresponding authors of this article.
This research was funded by National Natural Science Foundation of China and supported by the SINH Core Facilities.
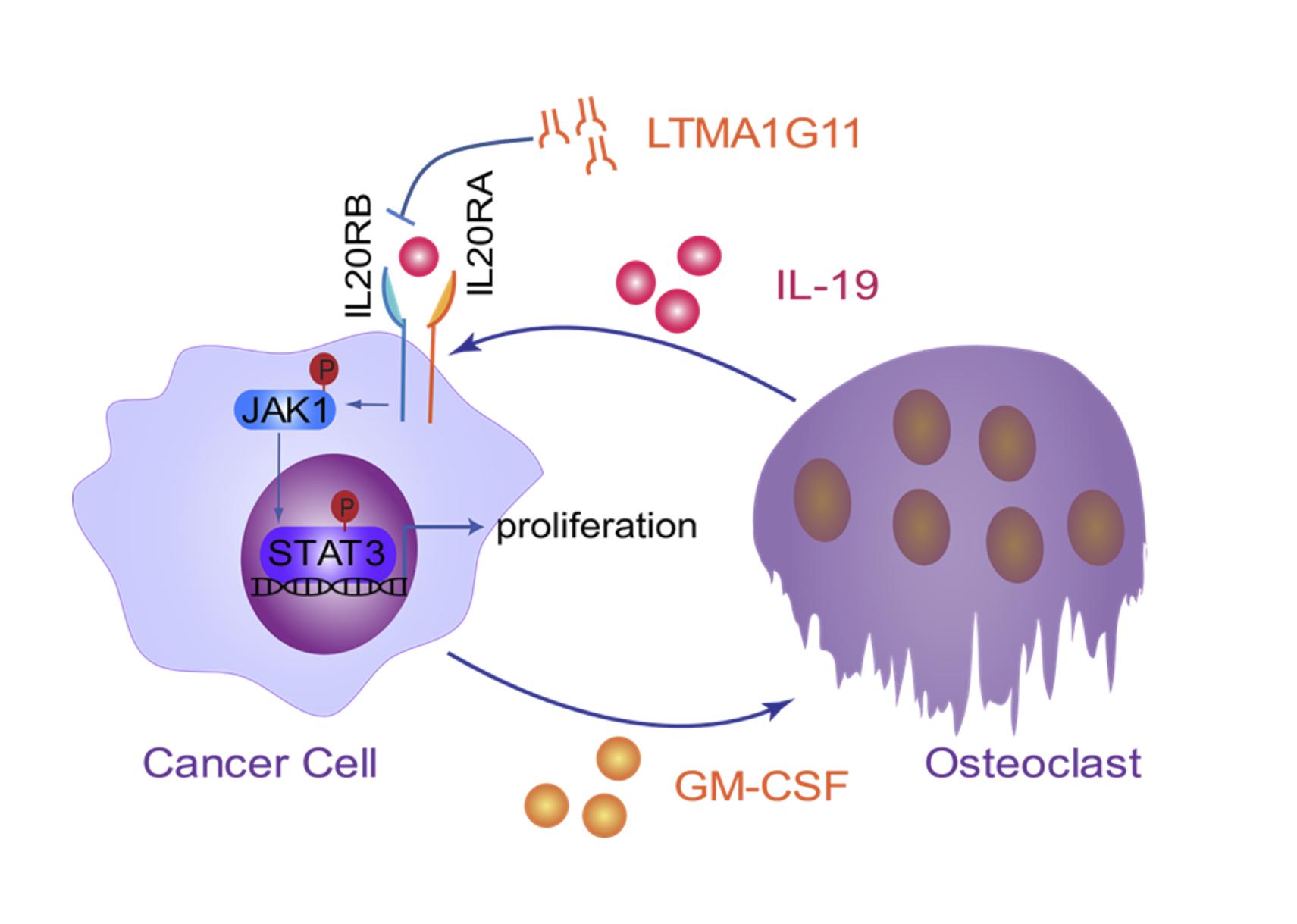
A schematic model of the IL19-IL20RB axis in lung cancer bone metastasis. (Image provided by Prof. HU Guohong’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

