A recent study led by Dr. WANG Lan’s group from Shanghai Institute of Nutrition and Health(SINH) of the Chinese Academy of Sciences(CAS) reveal the role and mechanism of UHRF1 in acute myeloid leukemia (AML), elucidating the mechanism of UHRF1 in the development and maintenance of AML and discovering a specific UHRF1 inhibitor. This research provides a theoretical basis and potential diagnosis strategies for the clinical treatment of AML. This work entitled “Targeting UHRF1-SAP30-MXD4 axis for leukemia initiating cell eradication in myeloid leukemia” was published online in Cell Research on Oct. 27th, 2022.
AML is a clonal disease of abnormal hematopoietic stem/progenitor cells characterized by an increase in immature myeloid cells in the bone marrow. It is the most common acute leukemia in adults, and an overall five-year survival rate 25-40 percent for AML patients. The prognosis is worse for older patients and those with relapsed or refractory AML who are not candidates for intensive chemotherapy. While chemotherapy remains the primary treatment strategy for many AML patients, conventional chemotherapy for AML patients does not effectively target the AML-initiating cells. Therefore, new therapeutic strategies are urgently needed to target the self-renewal of AML leukemia initiating cells.
In this study, the researchers found that UHRF1 was significantly higher in AML patients, that high UHRF1 expression was associated with a poorer prognosis in AML patients. Uhrf1 deficiency significantly delayed the onset, maintenance, and progression of AE9a or MLL-AF9-driven AML in mice and significantly inhibited the self-renewal of AML initiating cells using a mouse model of Uhrf1 conditional knockout. Knocking down UHRF1 significantly led to proliferation suppression, increased apoptosis and cell cycle arrest in AML cells.
Mechanistically, by analyzing RNA-seq data from AML cells, the researchers found that the knockdown of UHRF1 significantly enriched signaling pathways such as MYC, E2F and G2/M checkpoints. Combining RNA-seq, CUT&Tag, and bisulfite sequencing analyses, the researchers found that MXD4 is a direct target of UHRF1 by regulating its DNA methylation, and that knocking down UHRF1 reduces the DNA methylation level of MXD4, leading to high expression of MXD4 in mRNA and protein levels. As an antagonist of MYC, high expression of MXD4 can significantly inhibit MYC’s downstream signaling pathways. In addition, knocking down Mxd4 significantly restored the reduction in leukemia-initiating cells and the delayed survival of AML caused by Uhrf1 knockdown.
To further understand the role of UHRF1 in leukemogenesis, researchers performed the mass spectrometry analysis and Co-IP, which revealed that UHRF1 interacts with SAP30. GST-Pull down analysis showed that the UHRF1-SRA domain had a direct interaction with SAP30. Immunoprecipitation experiments using the truncation of UHRF1 showed that G572 and F573 on the SRA domain were able to interact with SAP30. The UHRF1 mutation significantly decreased the DNA methylation level of MXD4 and led to high expression of MXD4. Moreover, mutations in UHRF1 significantly impaired the ability of UHRF1 to rescue AML from self-renewal and leukemogenesis.
To find a specific UHRF1 inhibitor, the researchers performed a screening using molecular docking and deep learning. Surface plasmon resonance analysis and the fluorescence resonance energy transfer analysis showed that UF146 significantly inhibits the binding of UHRF1 and hemi-methylated DNA. UF146 significantly inhibited the proliferation and self-renewal of mouse AML initiating cells and primary AML patient cells. Moreover, UF146 significantly delayed the development of AML, with lower white blood cells counts and fewer leukemic blasts in peripheral blood. In addition, analysis of AML patient cell-derived xenograft mouse models showed that UF146 significantly prolonged the survival of the recipients.
In conclusion, this study has revealed that UHRF1 is required for the occurrence and development of AML by maintaining self-renewal of LICs, and that UHRF1 directly interacts with SAP30 to repress the expression of the target gene MXD4 by maintaining DNA methylation. UHRF1 inhibitors have demonstrated significant efficacy in mouse models of myeloid AML.
This work was conducted collaboratively by Prof. WANG Lan's team at SINH, Prof. ZHENG Mingyue's team at Shanghai Institute of Materia Medica of CAS, Prof. SUN Xiao-Jian's team at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine(SJTUSM) and Prof. LIU Xiaolong's team at Center for Excellence Molecular Cell Science of CAS. Postdoctoral researchers Dr. HU Cheng-Long and Dr. CHEN Bing-Yi, PhD students LI Zijuan, YANG Tianbiao, and Dr. XU Chun-Hui are the co-first authors of this work.
This work has been strongly supported by collaborators in several medical and scientific research institutions, including Prof. CHEN Saijuan and CHEN Zhu at Ruijin Hospital of SJTUSM, Prof. SHEN Shuhong at Shanghai Children's Medical Center of SJTUSM, Prof. CHANG Chunkang and Prof. WU Lingyun at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Prof. ZHANG Qunling at Fudan University Shanghai Cancer Center.
This work was supported by National Natural Science Foundation of China, Ministry of Science and Technology, CAS and State Key Laboratory of Medical Genomics.
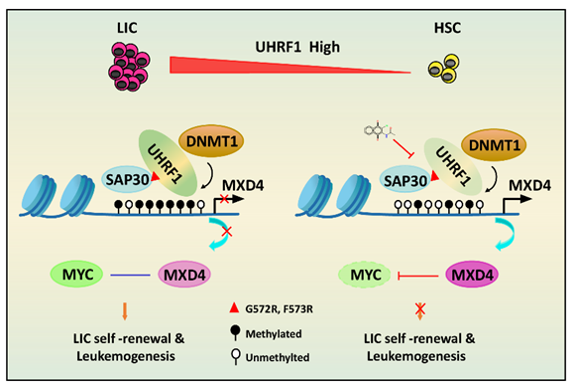
The molecular mechanism of UHRF1 in myeloid leukemogenesis. (Image provided by Dr. WANG’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

