A recent study led by Dr. WANG Lan’s group from Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences (CAS) revealed the cell non-autonomous role and mechanism of ID1 in acute myeloid leukemia (AML), illustrating the mechanism of the inhibitor of DNA binding 1 (ID1) in the initiation and development and of AML cell-non-autonomously. This research provided a novel therapeutic strategy for the clinical treatment of AML.
This work entitled “The cell non-autonomous function of ID1 promotes AML progression via ANGPTL7 from the microenvironment” was published online in Blood on June 15, 2023.
AML is a type of malignant tumor originating from hematopoietic stem/progenitor cells (HSPCs), and is characterized by enhanced clonal expansion and differentiation block of immature myeloid cells in the bone marrow. AML is the most common form of adult leukemia, and its high heterogeneity limits prognosis and outcomes. While clinical governance of AML has improved significantly over the past few years, significant challenges remain, such as the overall survival time for 50% of AML patients is still less than 4 years, and the heterogeneity of AML cells also leads the recurrence in 50% of patients. Therefore, there is an urgent need to improve our understanding of the mechanisms by which AML occurs, and to develop new therapeutic strategies.
The initiation and development of AML is often accompanied by abnormal gene expression. WANG’s lab previously uncovered that ID1 is a target gene for AML1-ETO, a leukemogenic fusion protein, and that ID1 interacts directly with AKT1 to promote AKT1 phosphorylation, thereby accelerating the initiation and progression of leukemia. Previous work by the same team have shown the differential action of Id1 in MLL-AF9-driven leukemia based on cell of origin. Id1 ablation in fetal liver HSPCs significantly weakens the development of leukemia, whereas Id1 loss in bone marrow HSPCs accelerates leukemogenesis.
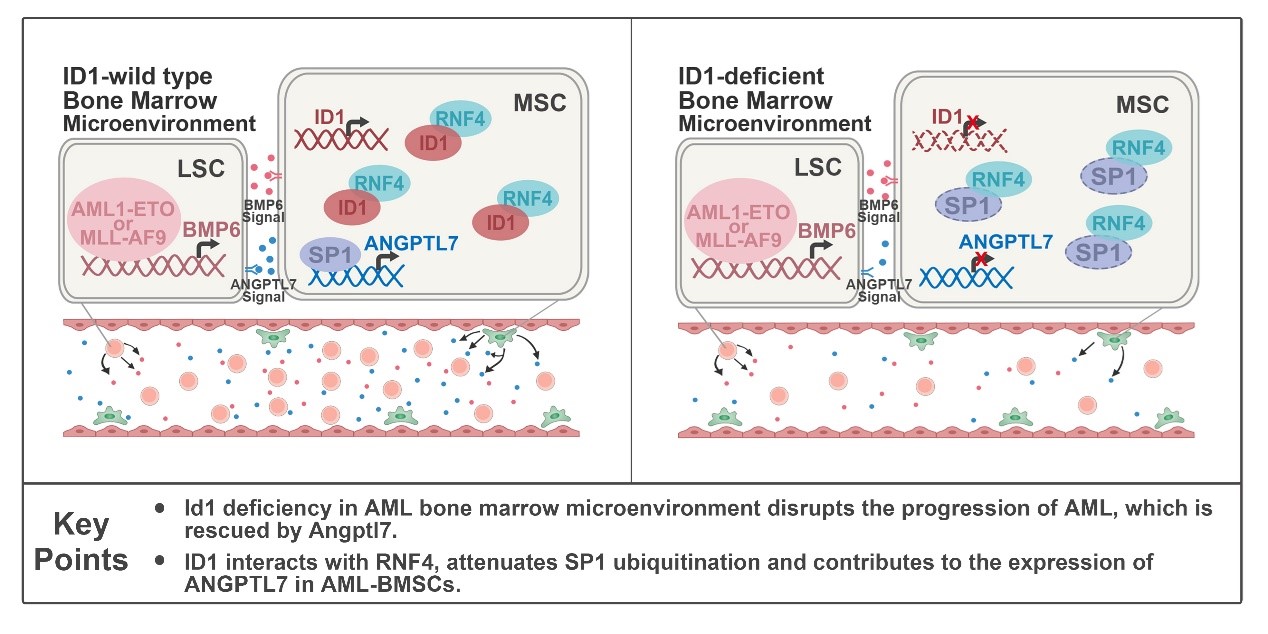
In this study, the researchers found that ID1 is highly expressed in Mesenchymal Stem Cells (MSCs) derived from AML patients, and especially in AML subtypes M2 and M5. To investigate the potential mechanism of ID1 upregulation in MSCs derived from AML patients, the researchers performed ChIP-seq analysis and found that AML1-ETO and MLL-AF9 directly bind to the promoter region of BMP6. The researchers used shRNA to knock down BMP6 in Kasumi-1 and THP-1 cells and observed significant downregulation of ID1 protein levels in HS-5 cells co-cultured with these cells, suggesting that AML cells promote the ID1 expression in stromal cells through BMP6 secretion. ID1 deletion in host BMM delayed the onset and progression of AML1-ETO9a or MLL-AF9-driven AML in mice and significantly inhibited the self-renewal of Leukemia Stem Cells (LSCs ). Knocking out of ID1 in mesenchymal cells significantly suppresses the proliferation of co-cultured AML cells.
Mechanistically, by analyzing bone marrow supernatant fluid (BMSF) of Id1+/+ and Id1-/- recipients with AML, researchers found a significant downregulation of Angptl7, a member of the secreted glycoprotein family that supports HSPCs expansion, in the BMSF of Id1-/- recipients with AML. To further examine that ANGPTL7 is regulated by ID1 in the Bone Marrow Microenvironment (BMM ), researchers performed in vitro co-culture assays and supplemented with recombinant ANGPTL7 in ID1-/- HS-5 cells, which significantly rescued proliferation suppression and cell cycle arrest in co-cultured AML cells. To assess the function of Angptl7 in the progression of AML in vivo, the researchers attempted an intra-BM transfusion of Angptl7 to the recipient. As a result, intra-BM transfusion of Angptl7 significantly accelerated AML progression in Id1-/- recipients.
To further understand the cellular non-autonomous role of ID1 in leukemogenesis, the researchers performed mass spectrometry analysis and GST-Pull down assays, which revealed that ID1 interacts with RNF4, a ubiquitin E3 ligase of SP1. Co-IP analysis showed that the interaction between the C terminal of ID1 and the SIM region of RNF4 weakens the ubiquitination of SP1, followed by an upregulation of ANGPTL7. Taken together, ID1 interacts with RNF4 to inhibit SP1 degradation, which contributes to AML progression non-cell-autonomously.
In summary, this study has revealed that ID1 regulated ANGPTL7 expression is required for the occurrence and development of AML cell-non-autonomously by maintaining self-renewal of LSCs, and also revealed that AML cells promote ID1 expression in the BMM by secreting BMP6. More importantly, ID1 controls the initiation and progression of leukemia in a cell-autonomous role, and the regulatory loop of ID1 between MSCs and LSCs has already demonstrated the importance of ID1 as a therapeutic target for AML.
This work has been strongly supported by collaborators in several medical and scientific research institutions, including Prof. SUN Xiaojian and Xue Kai at Ruijin Hospital of Shanghai Jiao Tong University School of Medicine (SJTUSM), Prof. CHANG Chunkang and Prof. WU Lingyun at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Prof. SHEN Shuhong at Shanghai Children's Medical Center of SJTUSM, Prof. YAN Jinsong at The Second Hospital of Dalian Medical University, Prof. ZHANG Qunling at Fudan University Shanghai Cancer Center.
This work was supported by the National Key Research and Development Plan of China, the National Natural Science Foundation of China General Program, Shanghai “Science and Technology Innovation Action Plan” Excellent Academic/Technical Leader Program (Youth).
The molecular mechanism of cell non-autonomously role of ID1 in myeloid leukemogenesis.
(Image provided by Dr. WANG Lan’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

