As living organisms are semi-open systems, they require barriers to control the transport and exchange of matters. In animals, gut epithelial cells are tightly anchored through a series of cell membrane fusion proteins, forming "tight junctions" to seal the intercellular space to control the intake of nutrients while preventing their leakage. Root is an organ of plants for water and nutrient uptake and transport, and it structurally and functionally resembles the animal gut. Root endodermal cells, resembling gut epithelial cells, function in controlling water/nutrient transport and preventing water/nutrient leak. However, due to the presence of the cell wall, plant root endodermal cells cannot establish direct tight junctions like gut epithelial cells. Over a long period of evolution, plants have developed a unique cell wall structure called the Casparian strip that prevents free diffusion of water and nutrients. Named after its discoverer Robert Caspary, this structure is composed of highly hydrophobic lignin, which prevents the free diffusion of water-soluble substances. In 1935, American scientist Bryant discovered that the plasma membrane next to the Casparian strip, so called Casparian strip domain (CSD), tightly adheres to the Casparian strip, resembling tight junctions in animals. Such an adhesion is incredibly strong and cannot even be separated by high osmotic treatments that tear cells apart. For a long time, this adhesion has been speculated to play an important role in plants. Nevertheless, owing to the difficulty of research and lack of evidence, this speculation has not been confirmed, and the molecular basis and adhesion mechanism it produces have remained unclear.
This study first identified a series of genes specifically expressed in the root endodermis of rice through bioinformatics analysis. Among them, three previously uncharacterized genes were highly similar in sequences, and the proteins they encode have a C-terminal non-conserved structure rich in glycine (G), alanine (A), and proline (P), and a conserved lectin domain (LE) and a secretion signal peptide (SS) at the N-terminus. The researchers thus named the family GAPLESS. These proteins are widely present in plants and specifically expressed in unsuberized cells of the endodermis.
When the genes encoding the three GAPLESS proteins were individually knocked out, only the mutant gapless1 exhibited obvious growth and ion homeostasis defects, such as reduced plant height, decreased tillering, lower individual yield, reduced potassium ion levels, and increased calcium ion levels. However, the double mutant gapless1/2 showed extremely severe growth and ion homeostasis phenotypes, with a yield less than 3% of the wild type and potassium ion content only 10% of the wild type. These results indicate the critical importance of these proteins for rice and suggest functional redundancy of these genes.
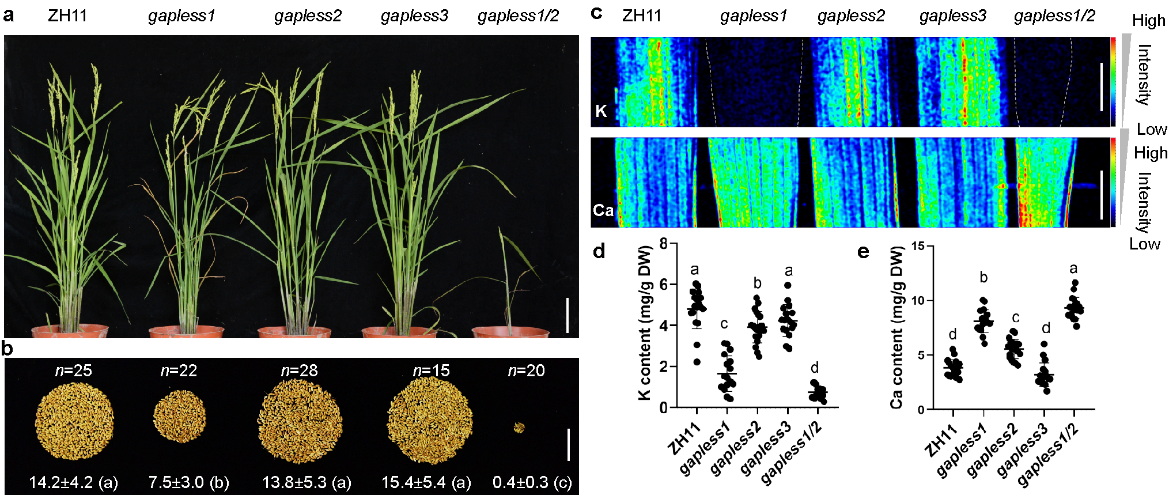
Figure 1. GAPLESS proteins are required for rice growth, yield and nutrient homeostasis.
Subsequent research showed that the barrier function of the root endodermis of the single mutant gapless1 to prevent the free diffusion of water-soluble molecules decreased, while that in the double mutant gapless1/2 was even more severely impaired. However, further investigation revealed that their Casparian strip lignin accumulation was not defective; instead, the Casparian strip in the gapless1/2 double mutant was wider and stronger. This suggests that their barrier defects are not caused by Casparian strip defects. As the adhesion of CS and CSD is necessary for establishing the endodermal cell barrier, the researchers used electron microscopy to observe this structure in wild-type and mutant plants. The results showed that the adhesion of CS and CSD weakened in the gapless1 single mutant, and about half of the adhesion was completely lost in the gapless1/2 double mutant, with even the remaining adhesion severely narrowed. These findings indicate that the GAPLESS protein family is necessary for CS and CSD adhesion and that this cell adhesion is crucial for nutrient balance and growth development in rice.
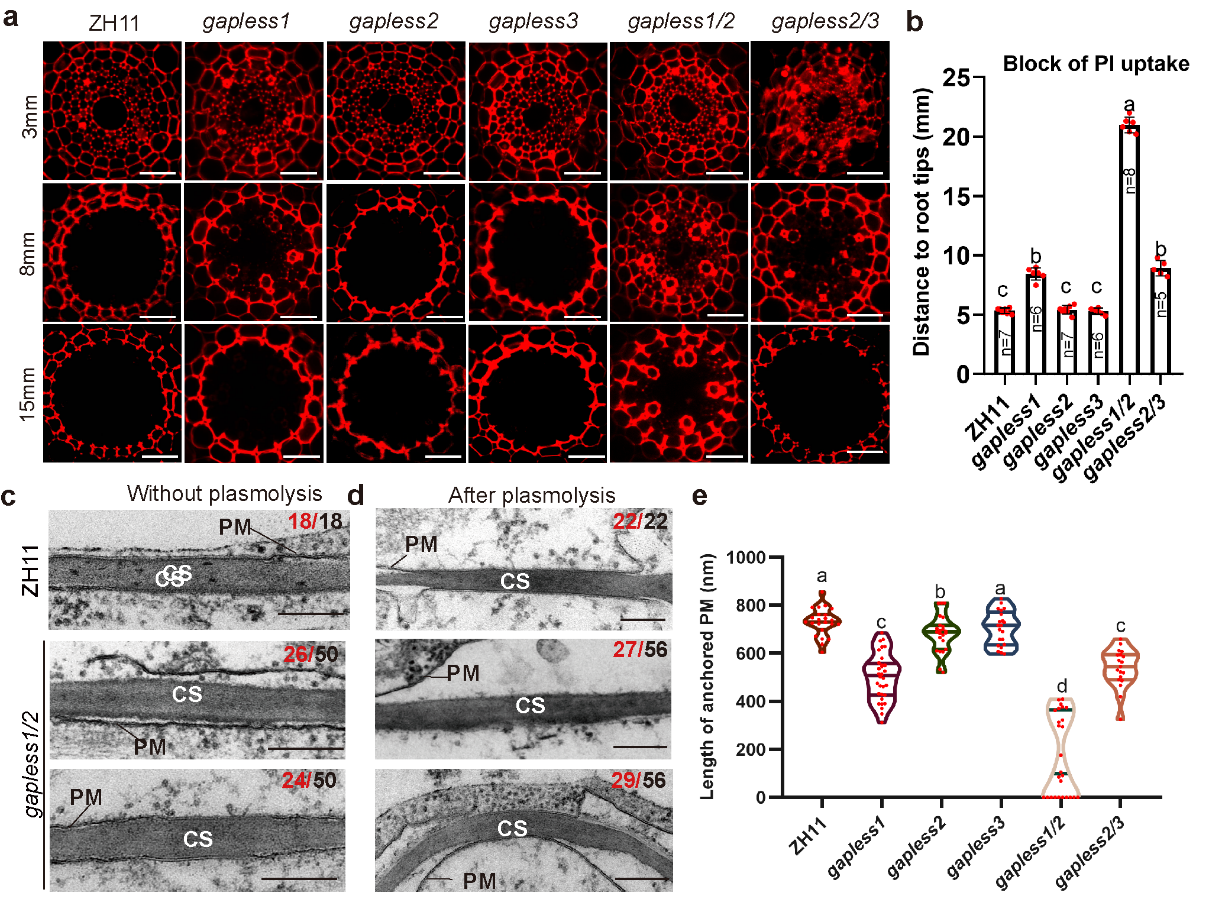
Figure 2. The family of GAPLESS proteins is required for apoplastic diffusion.
Further investigation revealed that the GAPLESS proteins are specifically localized in CS and close to the OsCASP1 protein, which is specifically localized in the CSD. A series of experimental evidence showed that the GAPLESS protein can strongly interact with OsCASP1, forming a robust GAPLESS-OsCASP complex. Therefore, the GAPLESS protein embedded in the CS and OsCASP embedded in CSD to form a stable complex, adhering the Casparian strip to the cell membrane, thereby preventing the free diffusion of water and ions in the root.
The elucidation of the molecular features and mechanism of action of GAPLESS not only refreshes understanding of the function of cell wall proteins but also expands our knowledge of cell adhesion mechanisms in multicellular organisms. This research unravels the century-old mystery of the molecular mechanism underlying the formation of the Casparian strip-cell membrane adhesion and confirms the crucial importance of this tight adhesion for plant nutrient balance and growth development. Additionally, since the Casparian strip plays a vital role in plant selective absorption and response to stresses like drought and salinity, this study also holds significant implications for improving the efficiency of mineral nutrient utilization and deciphering plant salt and drought tolerance mechanisms.
Figure 3. GAPLESS proteins mediate adhesion of CS by forming a complex with OsCASP1 in CSD
Prof. Dai-Yin Chao is the corresponding author of this paper, and PhD student Song Tao is the first author. This research was supported by the National Natural Science Foundation of China, Chinese Academy of Sciences, and the Newton fund.
Link: https://www.nature.com/articles/s41477-023-01503-z
Contact: Dr. Dai-Yin Chao, Professor
National Key Laboratory of Plant Molecular Genetics, Center for Excellence in Molecular Plant Sciences, Chinese Academy of Sciences
E-mail: wjiang@cemps.ac.cn

