Mutations in X-linked gene MeCP2 (methyl CpG binding protein 2) are known to be the major cause of Rett syndrome (RTT), a severe neurodevelopmental disorder occurs in 1 of every ~10,000 female births. The affected female babies may develop normally for the first 6-18 months before the onset of progressive mental and physical deterioration which is followed by premature death. MeCP2 is known to function as an important reader of epigenetic signals by binding to methylated genomic DNA. Highly elevated levels of glutamate have been detected in the cerebrospinal fluid and brains of RTT patients which has been proposed to contribute to neural dysfunction. However, there are still many aspects of this devastating disorder that we still do not understand, nor do we have an effective treatment for this devastating disorder.
Recently, a research group led by Professor YUAN Junying at the Interdisciplinary Research Center on Biology and Chemistry (IRCBC), Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, reported in PNAS that "RIPK1 activation in Mecp2 deficient microglia promotes inflammation and glutamate release in RTT" (Cao et al. PNAS. 2024). This study reveals the role of RIPK1 kinase in the onset and progression in MeCP2 null mice, a mouse model of RTT. Cao et al. show that MeCP2 deficiency in microglia promotes oxidative stress and neuroinflammation which can be effectively inhibited by inhibition of RIPK1. Activation of RIPK1 in MeCP2 deficient microglia promotes the release of excess glutamate which can impair neural dysfunction. Thus, the activation of RIPK1 in MeCP2 deficient microglia links the inflammatory response in microglia to glutamate mediated excitotoxicity in neurons. Genetic inhibition of RIPK1 kinase ameliorates the development of motor dysfunction and prolongs the survival of MeCP2 null mice. Furthermore, oral administration of RIPK1 inhibitor Nec-1s moderates the disease progression after the onset of motor dysfunction in MeCP2 null mice.
This study suggests the possibility of using a RIPK1 inhibitor to treat RTT in early stages of the disease development.
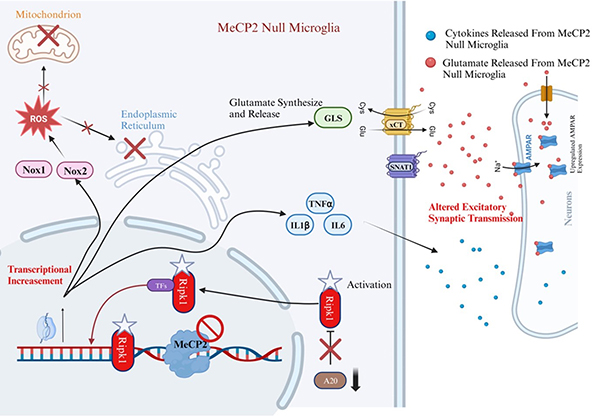
Fig 1 A schematic model for the mechanism by which Mecp2 deficiency promotes RIPK1-mediated microglial activation in cell autonomous manner and neuronal dysfunction cell non-autonomous manner. (Image by YUAN Junying)
This work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology Commission.
YUAN Junying Ph.D., Professor
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Haike Road 100 Shanghai 201210 China
Tel: 0086-21-6858238
Email: junying_yuan@sioc.ac.cn

