|
|
 |
Research Progress |
| New Progresses in Elucidating the Structural Mechanism of Autophagy Receptor TAX1BP1 |
Autophagy is an important catabolic process necessary for the normal operation of almost all mammalian cells, and plays an important role in many physiological processes including maintaining cellular homeostasis, embryonic development, innate immunity, and aging. Meanwhile, the dysfunction of autophagy is closely related to many other human diseases, such as cancer and neurodegenerative diseases. As a multifunctional and ubiquitin-binding autophagy receptor, TAX1BP1 plays a crucial role in the selective autophagy of invading pathogens, protein aggregates, and damaged lysosomes. However, many of the detailed molecular mechanism governing the specific recruitments of relevant upstream autophagy machinery and ATG8 family proteins by autophagy receptor TAX1BP1 during selective autophagy are still poorly understood.
Recently, a research paper titled “Mechanistic insights into the interactions of TAX1BP1 with RB1CC1 and mammalian ATG8 family proteins” was published on the PNAS journal by Professor PAN Lifeng’s group from Shanghai Institute of Organic Chemistry, CAS (https://www.pnas.org/doi/10.1073/pnas.2315550121). In this paper, they have systematically characterized the interaction between TAX1BP1 and RB1CC1 (one of the autophagy initiation ULK complex subunits), and discovered, for the first time, that TAX1BP1 binds to RB1CC1 with a dual-site interaction mode. In addition to the known SKICH domain, which can specifically bind to RB1CC1 coiled-coil domain, TAX1BP1 coiled-coil domain can also interact with the C-terminal region (CTR) of RB1CC1. The dissociation constants determined by spectral shift assay suggest that the two binding sites do not act synergistically, thereby allowing RB1CC1 and TAX1BP1 to form oligomers. Subsequently, they have elucidated the detailed binding mechanism for the interaction of RB1CC1 with TAX1BP1 by solving the crystal structure of the TAX1BP1 SKICH/RB1CC1 coiled-coil complex. Based on their previously resolved structure of TAX1BP1 SKICH/NAP1 complex (PNAS. 2018, 115, E11651-E11660), they have found that TAX1BP1 SKICH adopts a completely different region to bind to RB1CC1 and NAP1. Interestingly, subsequent studies have revealed that RB1CC1 coiled-coil and NAP1 coiled-coil are competitive in binding to the TAX1BP1 SKICH domain due to allosteric effects. Additionally, the introduction of the NAP1 FIR motif can facilitate the formation of the stable RB1CC1/TAX1BP1/NAP1 ternary complex. Further relevant biochemical assays have corroborated the binding mode of the RB1CC1/TAX1BP1/NAP1 ternary complex, and revealed that NAP1 recruits both RB1CC1 and TAX1BP1 simultaneously through its FIR motif and coiled-coil domain, respectively. Moreover, they have determined the structure of GABARAP in complex with the non-canonical LIR motif (CLIR) of TAX1BP1, revealing the molecular mechanism by which TAX1BP1 selectively recognizes mammalian ATG8 family proteins through its CLIR motif. Finally, they have also found that the individual TAX1BP1, the TAX1BP1/RB1CC1 binary complex, and the TAX1BP1/NAP1/RB1CC1 ternary complex have different binding abilities for interacting with ATG8 family proteins, and explored the potential mechanisms through related biochemical experiments and structural modeling analyses.
In conclusion, they have systematically investigated the molecular mechanism of autophagy receptor TAX1BP1 for binding to ATG8 family proteins, the RB1CC1 subunit of the autophagy initiation ULK complex as well as its associated protein interaction network, and revealed the unique molecular mechanism of TAX1BP1 for binding to ATG8 family proteins and RB1CC1, for the first time, and clarified the inter-relationship between TAX1BP1, RB1CC1, NAP1, and ATG8 family proteins. These findings provide a good foundation for further understanding the molecular mechanism of TAX1BP1-mediated selective autophagy.
The PhD student ZHANG Mingfang from Professor PAN’s group is the first author of this paper. This work was supported by grants from the National Natural Science Foundation of China (32071219, 92253301, 21822705), the CAS Youth Interdisciplinary Team (JCTD-2022-10), the National Basic Research Program of China (2022YFC2303102), and the Science and Technology Commission of Shanghai Municipality (20XD1425200).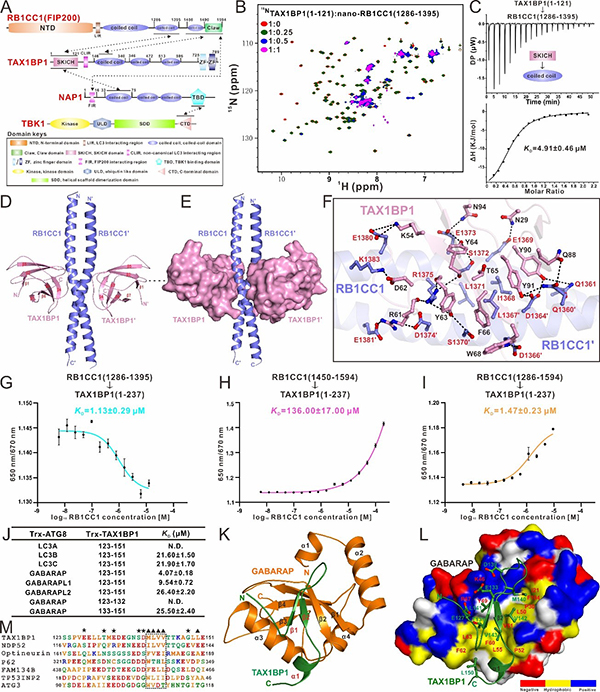
Figure 1. Mechanistic insights into the interactions of TAX1BP1 with RB1CC1 and ATG8 family proteins
PAN Lifeng Ph.D.Professor Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences Ling Ling Road 345 Shanghai 200032 China Tel: 0086-21-54925561 Email: panlf@sioc.ac.cn |
|
|

