A research team led by Prof. LI Yu from the Shanghai Institute of Nutrition and Health (SINH) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. XU Yong from the Affiliated Hospital of Southwest Medical University, identified a novel mechanism for CBP/p300 and HDAC3-mediated reversible acetylation of CREB/ATF bZIP transcription factor (CREBZF) in the regulation of thermogenesis and energy homeostasis in adipose tissue. Entitled “Glucose regulation of adipose tissue browning by CBP/p300-and HDAC3-mediated reversible acetylation of CREBZF”, this study was published online in Proceedings of the National Academy of Sciences (PNAS) on April 8th, 2024.
Adipose tissues are sufficient to store surplus energy or dissipate energy through thermogenesis. Under cold circumstances, glucose uptake is elevated in brown and beige adipocytes, and glucose functions as the energy supply for thermogenesis. However, whether glucose signals are involved in the regulation of thermogenesis is still unknown.
This study demonstrated that glucose signals are essential for the regulation of thermogenic program in beige adipocytes. CREBZF protein is potently induced by glucose and mediates its effects on energy homeostasis in adipose tissues.
To investigate the roles of glucose in regulating the activity of thermogenic program in adipocytes, mice were treated with glucose injection under cold conditions. After glucose treatment, mice showed enhanced browning of white adipose and resistance to cold stress. Protein levels of CREBZF were stimulated by glucose in beiges. Surprisingly, adipose-specific CREBZF deficiency further increased the glucose-induced thermogenesis and browning.
Mechanistically, glucose increases the protein stability of CREBZF through transacetylase CBP/p300-mediated acetylation modification of K208 site on CREBZF protein. In contrast, deacetylase HDAC3 functions to remove the acetyl of K208 site. Glucose-induced CREBZF further interacts with PGC-1α and inhibits the activity of thermogenic program.
In physiological conditions, glucose-induced CREBZF may protect beige adipocytes from hyperactivation and unnecessary energy dissipation. In addition, glucose-dependent activation of CREBZF may represent the mechanism for reduced thermogenic capacity and energy expenditure in the pathological conditions, such as hyperglycemia insulin resistance and obesity.
These findings advance the understanding of glucose signals in regulating energy metabolism in adipose, and the reversible acetylation of CREBZF controlled by glucose provides a fine-tuned regulatory mechanism that couples nutrient signals to energy homeostasis and browning of adipose tissues.
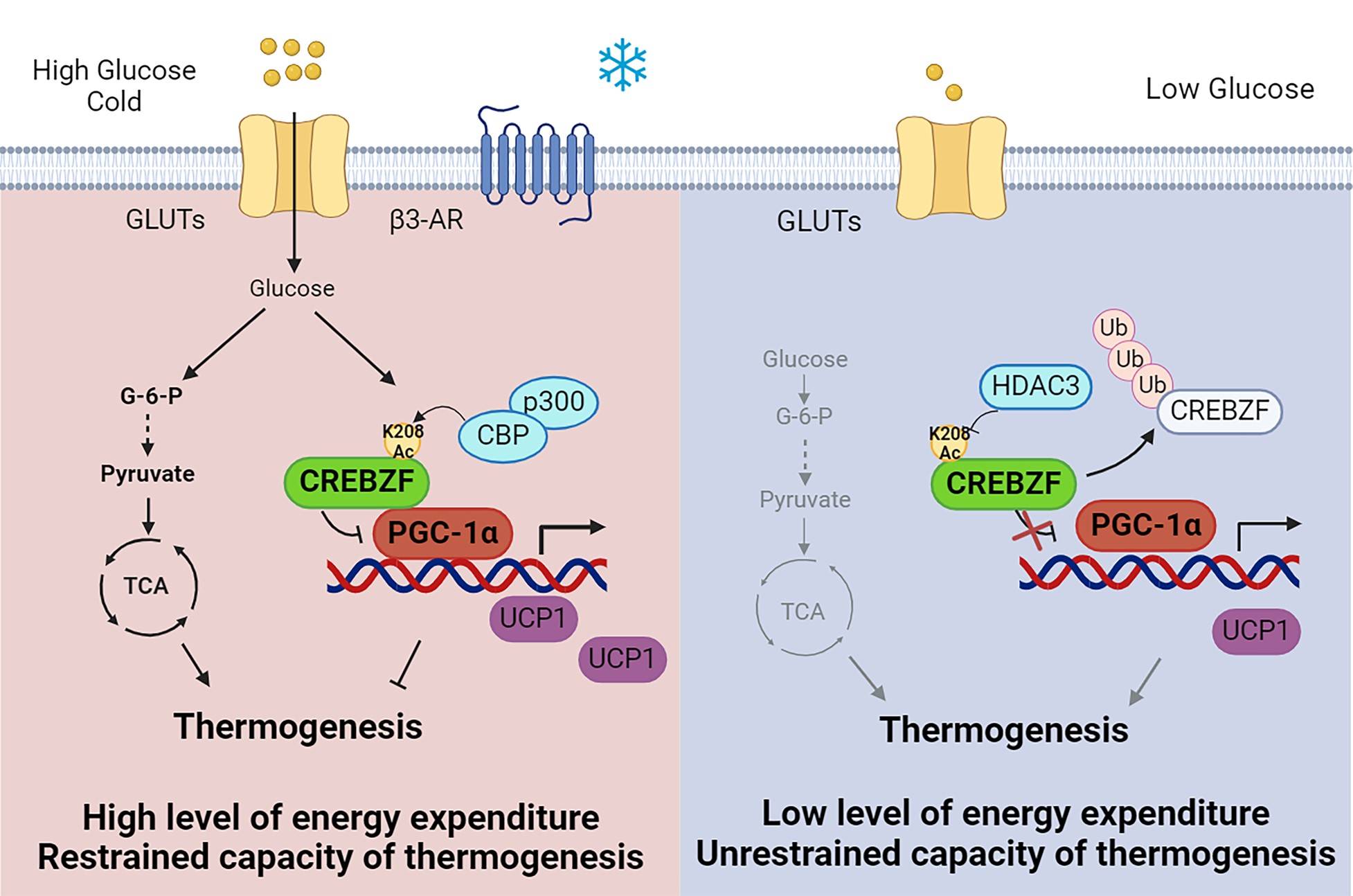
The proposed model for glucose sensing via CREBZF in the regulation of thermogenesis and energy homeostasis in adipose tissues. Targeting CREBZF may provide novel therapeutic avenues for the treatment of obesity and related metabolic disorders.
(Image provided by Prof. LI Yu’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

