Researchers led by Prof. XU Daichao from the Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry have uncovered the molecular link between glucose sensing and non-classical pyroptosis.The nutritional status and pyroptosis play a crucial role in the host’s defense against pathogens. Mounting evidence suggests that alterations in the nutritional status of host cells, particularly glucose levels, are critical for their response to pathogen infection. Host cell glucose levels may fluctuate during bacterial infection. This fluctuation may be part of the host’s immune defense and therefore beneficial, but it may also be an inevitable adverse consequence of infection. Pyroptosis is a regulated form of cell death that induces an inflammatory response in host cells, contributing to the host cell’s immune defense. However, the molecular link between host cell nutritional status and pyroptosis during microbial infection is still unclear.
AMPK is an intracellular serine/threonine kinase and a central energy sensor that responds to metabolic stress. It can be activated not only by sensing a decrease in intracellular energy status but also by sensing glucose levels. During microbial infection, host cells require a large amount of energy to drive the immune response. Therefore, most intracellular pathogen infections involve the activation of the host’s AMPK.
In this study, Prof. XU Daichao’s team found that in the early stages of Yersinia infection, Yersinia can induce a sharp increase in glycolysis levels in host immune cells, leading to a rapid and significant decrease in glucose levels within immune cells and mouse blood glucose levels, ultimately overactivating AMPK within the host (Fig. 1).
Under normal conditions, host cells activate the RIPK1-caspase-8-GSDMD-mediated non-classical pyroptosis pathway upon Yersinia infection to restrict its proliferation. However, Prof. XU Daichao’s team found that AMPK activated within host cells can directly inhibit RIPK1 through an inhibitory phosphorylation on the highly conserved S321 site, hereby restraining its kinase activity and subsequent caspase-8 activation, thus preventing pyroptosis (Fig. 1), and promoting unfavorable responses to infection.
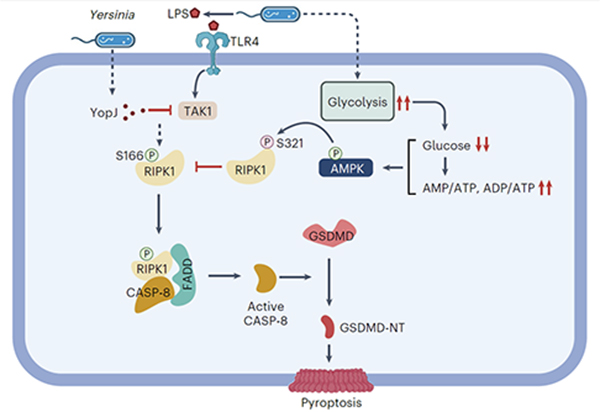
Figure 1. Molecular mechanism by which Yersinia escapes RIPK1-mediated pyroptosis by interfering with host cell glucose homeostasis to activate AMPK (image by XU Daichao)
Prof. XU Daichao’s team discovered that overactivating AMPK in mice, either through the use of AMPK agonists or methods such as glucose depletion during Yersinia infection, can worsen the severity of the infection. Conversely, knocking out AMPK in macrophages or supplementing glucose to inhibit AMPK activity during Yersinia infection in mice can significantly enhance the mice’s resistance to Yersinia infection.
In summary, this study investigated the impact of alterations in glucose homeostasis on the regulation of AMPK activation and RIPK1-dependent pyroptosis during Yersinia infection. Additionally, it elucidated the regulatory effect of pathogen infection on alterations in the nutritional status of host cells and their impact on the immune defense of host cells. The findings of this study suggest that maintaining patients’ nutritional status and blood glucose levels could be crucial in treating Yersinia infection.
This work was published in Nature Microbiology on June 6, 2024.
Paper link: https://www.nature.com/articles/s41564-024-01734-6.
XU Daichao Ph.D.Professor
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences
Haike Road 100, Shanghai, 201210 China
Tel: 0086-21-68582337
Email:xudaichao@sioc.ac.cn

