A team of researchers led by Prof. CHEN Yan and Prof. YANG Lifeng from the Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS), revealed a physiological function of monocarboxylate transporter 1 (MCT1) in mediating the intracellular and extracellular lactate transport in skeletal muscle. This study was published in Science Advances on June 26, 2024.
Researchers found that MCT1 regulates mitochondrial biogenesis of skeletal muscle and exercise activity. Deletion of MCT1 gene in the skeletal muscle promotes the production of oxidative muscle fibers, enhances exercise endurance, and improves metabolic phenotypes.
In the 1980s, Prof. George Brooks proposed the “lactate shuttle” theory, which posits that lactate serves as an energy substrate, produced by cells or tissues primarily relying on glycolysis for energy, but consumed by cells or tissues primarily utilizing oxidative metabolism. As the body's largest organ for energy metabolism, skeletal muscle is both a major producer and consumer of lactate. The transport of lactate in skeletal muscle depends on the MCT family of proteins, especially MCT1 and MCT4. As early as 1998, scientists discovered that the expression of MCT1 in skeletal muscle is positively correlated with its oxidative capacity, while MCT4 shows a negative correlation. Mammalian skeletal muscle mainly consists of four different types of muscle fibers, which are composed of different myosin types and metabolic characteristics. These muscle fibers include oxidative slow-twitch type 1 fibers, oxidative fast-twitch type 2A fibers, and glycolytic type 2X and 2B fibers. The metabolic characteristics of skeletal muscle depend on the proportion of these four types of muscle fibers. The higher the proportion of oxidative muscle fibers, the stronger the oxidative metabolic capacity of the skeletal muscle, and vice versa. However, the transport of lactate between different types of muscle fibers, as well as the specific distribution and function of MCT1 in different types of skeletal muscle fibers, have not yet been clearly elucidated.
In this study, researchers observed the specific distribution of monocarboxylate transporters MCT1 and MCT4 in skeletal muscle fibers, suggesting the possible existence of a “lactate shuttle” dependent on MCT1 and MCT4 between muscle fibers. Given the higher expression of MCT1 compared to MCT4 in skeletal muscle, the researchers constructed an animal model with muscle-specific knockout of MCT1 (mKO) to further investigate MCT1-mediated lactate metabolism in muscle fibers. The results showed that mKO mice exhibited improvement in exercise endurance, increase in the proportion of oxidative muscle fibers, and decrease in the proportion of glycolytic muscle fibers. At the metabolic level, using metabolic flux analysis and metabolomics combined with various animal experiments, researchers found that mKO mice had improved glucose tolerance, increased metabolic rate, and enhanced utilization of glucose in the tricarboxylic acid (TCA) cycle in skeletal muscle.
Mechanistically, the researchers proposed that lactate transport in skeletal muscle fibers depends on two shuttles, “cell-cell lactate shuttle” between muscle fibers and “intracellular lactate shuttle” within oxidative muscle fibers. This hypothesis was validated through analyses with the mKO mice. Normal lactate transport between muscle fibers depends on the combined involvement of MCT1 and MCT4. Lactate produced in glycolytic muscle fibers is transported extracellularly via MCT4, then taken up by oxidative muscle fibers through MCT1. Within oxidative muscle fibers, mitochondria also uptake lactate via MCT1, where mitochondrial lactate dehydrogenase (LDH) converts lactate to pyruvate, consuming equivalent amounts of NAD+, allowing pyruvate to enter the TCA cycle. In the absence of MCT1 in skeletal muscle, both lactate uptake by oxidative muscle fibers and its entry into mitochondria are impaired, leading to increased intracellular NAD+ levels. This increases the activity of SIRT1, an NAD+-dependent deacetylase, enhancing the deacetylation and activity of PGC-1α. Subsequently, this promotes the production of oxidative muscle fibers, mitochondrial biogenesis, as well as their activity and function, ultimately resulting in a series of changes in muscle functions.
This study revealed the physiological roles of lactate transport at the muscle fiber and cellular levels. It not only further validated and refined Prof. George Brooks' “lactate shuttle” theory but also sheded light on the physiological processes of skeletal muscle, providing a new theoretical basis for the study of skeletal muscle physiology and pathology. Moreover, the conversion of skeletal muscle fiber types has long been a hotspot in exercise physiology research. Given the different metabolic characteristics of muscle fiber types and their varying resistance to damage and aging, as well as their contributions to different forms of exercise, this study offered a new direction for research in metabolic improvements, pathological damage, and exercise physiology.
This study was supported by the National Key Research and Development Program of China Stem Cell and Translational Research, the National Natural Science Foundation of China, as well as the Shanghai Municipal Science and Technology Major Project, with acknowledgement of the assistance from the institutional animal facility and the public testing technical platform of SINH.
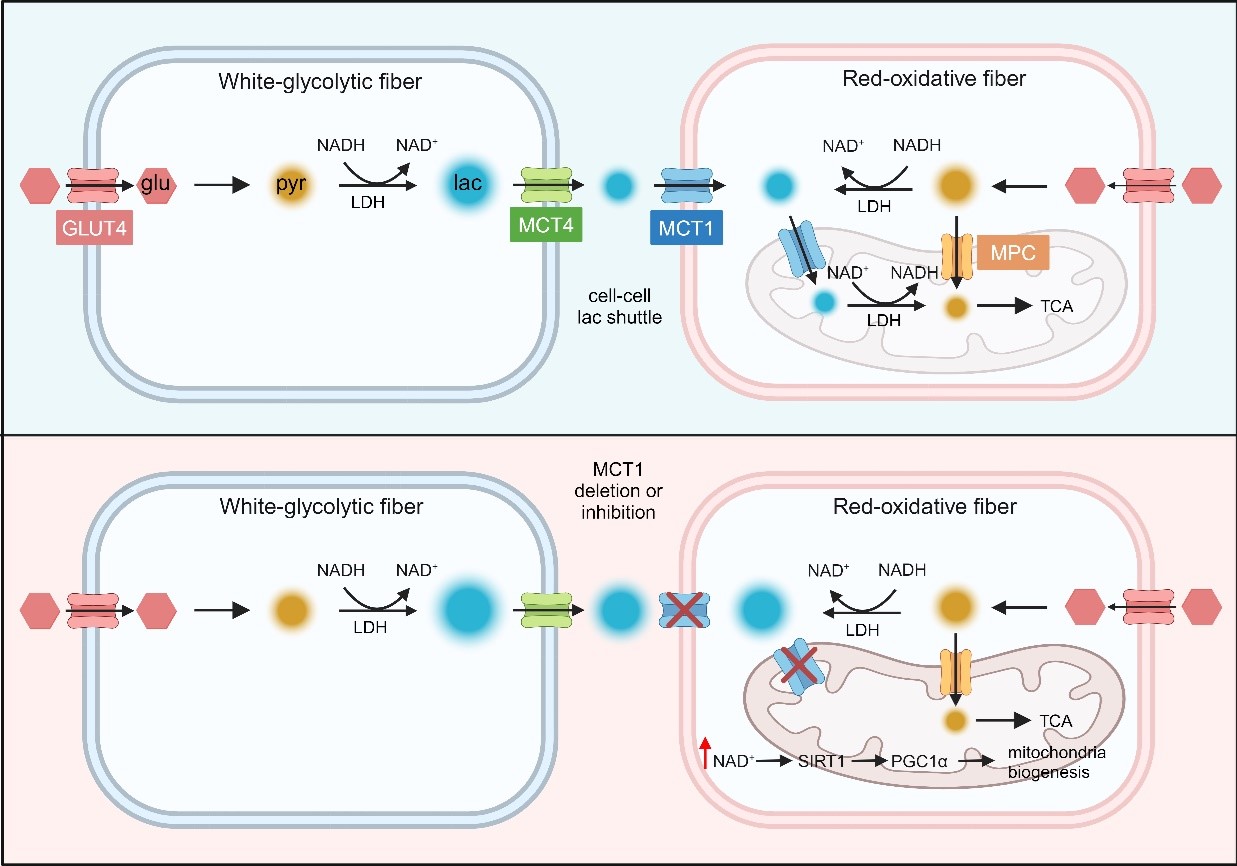
A model diagram of MCT1-mediated lactate shuttle in skeletal muscle. (Image provided by Prof. CHEN Yan’s group)
Media Contact:
WANG Jin
Shanghai Institute of Nutrition and Health,
Chinese Academy of Sciences
Email: wangjin01@sinh.ac.cn
Web: http://english.sinh.cas.cn/

